Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Modern Periodic Law
Modern Periodic Law
In 1913, a British Physicist Henry Moseley showed that the atomic number
is a more fundamental property of an element than its atomic weight. This
observation led to the development of modern periodic law. The modern periodic
law states that ' the physical and chemical properties of the elements are
periodic function of their atomic numbers.'
This means that when the elements are arranged in order of increasing
atomic numbers, the elements with similar properties recur after regular
intervals. The periodic repetition is called periodicity. The physical and
chemical properties of the elements are related to the arrangement of electrons
in the outermost shell. Thus, if the arrangement of electrons in the outermost
shell (valence shell) of the atoms is the same, their properties will also be
similar. For example, the valence shell configurations of alkali metals show
the presence of one electron in the s-orbital of their valence shells.
Similar behaviour of alkali metals is attributed to the similar valence
shell configuration of their atoms. Similarly, if we examine the electronic
configurations of other elements, we will find that there is repetition of the
similar valence shell configuration after certain regular intervals with the
gradual increase of atomic number. Thus we find that the periodic repetition of
properties is due to the recurrence of similar valence shell configuration
after certain intervals. It is observed that similarity in properties is
repeated after the intervals of 2, 8, 18, or 32 in their atomic numbers.
Long form
of the Periodic Table: The periodic table is
constructed on the basis of
repeating electronic configurations of the atoms when they are arranged in the
order of increasing atomic numbers. The long form of the Periodic table is
given in a modified form in page number 70. Readers are advised to follow the
periodic table closely while studying the structural features of the long form
of the Periodic Table.
Structural
Features of the Long form of the periodic Table: The long form of the periodic
table consists of horizontal rows called periods and vertical columns called
groups.
Periods: In terms of electronic structure of the atom, a period constitutes a series of elements whose
atoms have the same number of electron shell i.e., principal quantum number
(n). There are seven periods and each period starts with a different principal
quantum number.
The first period corresponds to the filling of electrons in the first
energy shell (n = 1). Now this energy level has only one orbital (1s) and,
therefore, it can accommodate two electrons. This means that there can be only
two elements (hydrogen, 1s1 and helium, 1s2 ) in the
first period.
The second period starts with the electron beginning to enter the second
energy shell (n = 2). Since there are only four orbitals (one 2s-and three 2p-
orbitals) to be filled, it can accommodate eight electrons. Thus, second period
has eight elements in it. It starts with lithium (Z = 3) in which one electron
enters the 2s-orbital. The period ends with neon (Z = 10) in which the second
shell is complete (2s22p6).
The third period begins with the electrons
entering the third energy shell (n = 3). It should be noted that out of nine
orbitals of this energy level (one s, three p and five d) the five 3d-orbitals
have higher energy than 4s-orbitals. As such only four orbitals (one 3s and
three 3p) corresponding to n = 3 are filled before fourth energy level begins
to be filled. Hence, third period contains only eight elements from sodium (Z =
11) to argon (Z = 18).
The fourth period corresponding to n = 4 involves the filling of one 4s
and three 4p-orbitals (4d and 4f orbitals have higher energy than 5s-orbital
and are filled later). In between 4s and 4p-orbitals, five 3d-orbitals are also
filled which have energies in between these orbitals. Thus, altogether nine
orbitals (one 4s, five 3d and three 4p ) are to be filled and therefore, there
are eighteen elements in fourth period from potassium (Z = 19) to krypton (Z =
36). The elements from scandium (Z = 21) to zinc (Z = 30) are called 3d-
transition series.
The fifth period beginning with 5s-orbital (n=5) is similar to fourth
period. There are nine orbitals (one 5s, five 4d and three 5p) to be filled
and, therefore, there are eighteen elements in fifth period from rubidium (Z =
37) to xenon (Z = 54).
The sixth period starts with the filling of
6s-orbitals (n= 6). There are sixteen orbitals (one 6s, seven 4f, five 5d, and
three 6p) in which filling of electrons takes place before the next energy
level starts. As such there are thirty two elements in sixth period starting
from cesium (Z = 55) and ending with radon (Z = 86). The filling up of 4f
orbitals begins with cerium (Z = 58) and ends at lutetium (Z = 71). It
constitutes the first f-inner transition series which is called lanthanide
series.
The seventh period begins with 7s-orbital (n =
7). It would also have contained 32 elements corresponding to the filling of
sixteen orbitals (one 7s, seven 5f, five 6d and three 7p), but it is still
incomplete. At present there are 23 elements in it. The filling up of 5f-
orbitals begins with thorium (Z = 90) and ends up at lawrencium (Z = 103). It
constitutes second f-inner transition series which is called actinide series.
It mostly includes man made radioactive elements. In order to avoid undue
extension of the periodic table the 4f and 5f- inner transition elements are
placed separately.
The number of elements and the corresponding
orbitals being filled are given below.
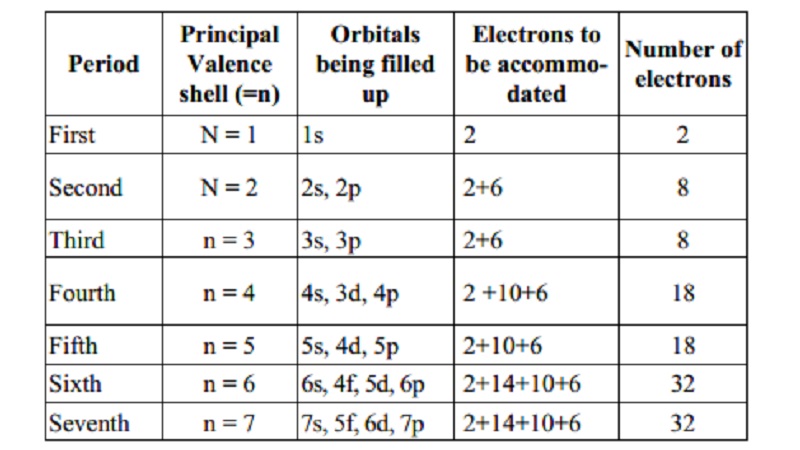
Principal Orbitals Electrons
to Number of
Period Valence being filled be accommo-
shell
(=n) up dated electrons
First N
= 1 1s 2 2
Second N
= 2 2s, 2p 2+6 8
Third n
= 3 3s, 3p 2+6 8
Fourth n
= 4 4s, 3d, 4p 2 +10+6 18
Fifth n
= 5 5s, 4d, 5p 2+10+6 18
Sixth n
= 6 6s, 4f, 5d, 6p 2+14+10+6 32
Seventh n
= 7 7s, 5f, 6d, 7p 2+14+10+6 32
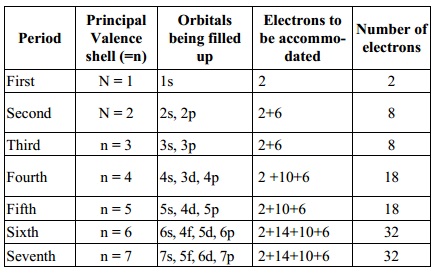
The first three periods containing 2, 8 and 8
elements respectively are called short periods, the next three periods
containing 18, 18 and 32 elements respectively are called long periods.
Groups
A vertical column in
the periodic table is known as group. A group consists of a series of elements having
similar configuration of the outer energy shell. There are eighteen vertical columns in long from of the periodic table.
According to the recommendations of the International
Union of Pure and Applied Chemistry (IUPAC),
these groups are numbered from 1 to
18. Previously, these were numbered from I to VII as A and B, VIII and zero
groups elements. The elements belonging to the same group are said to
constitute a family. For example, elements of group 17 (VII A) constitute
halogen family.
Related Topics