Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Isolation Of Noble Gases: Ramsay - Raleigh's and Dewar's Method
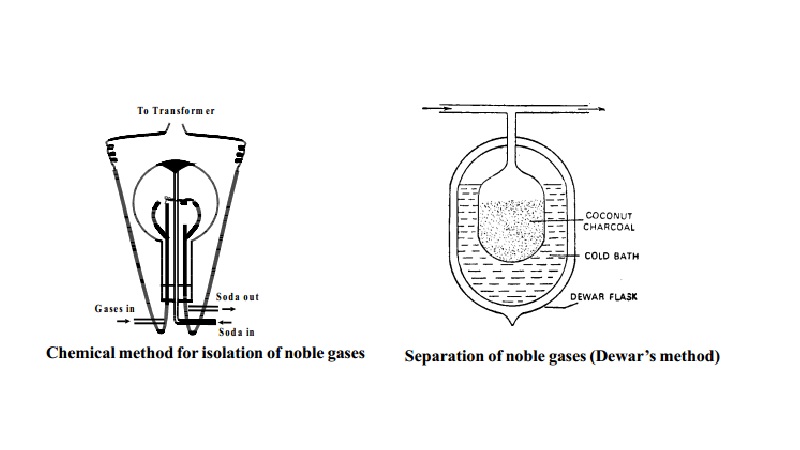
ISOLATION OF NOBLE GASES
The noble gases are isolated from air by removing oxygen
and nitrogen from air free from
carbon-di-oxide, water vapour, dust particles, etc., This can be accomplished by either chemical methods or physical
methods. In the chemical method, the
unwanted gases are removed by means of compound formation while in the physical method, these are removed by the
fractional evaporation of liquid air.
CHEMICAL METHOD
The first step in this method is to isolate the noble
gases mixed together, from the atmosphere
by passing repeated electric sparks in air so as to remove nitrogen and oxygen as nitrogen dioxide (N2 + 2O2 ® 2NO2 ). The second step
is to separate the various constituents from one another
taking advantage of the fact that they can
be adsorbed on activated charcoal at different temperatures.
Step 1 Removal of oxygen and nitrogen of the atmosphere as Nitrogen dioxide
Ramsay - Raleigh's method:- A mixture of air and oxygen is constantly admitted into a glass globe of about 50 litres capacity.
Two platinum electrodes are introduced and a discharge from a transformer of
about 6000 - 8000 volts is passed by the
action of which nitrogen and oxygen rapidly combine to form oxides of nitrogen. The oxides are dissolved out in a
solution of sodium hydroxide continuously
circulated through the flask.
N2+ O2 ® 2 NO
2 NO + O2 ® 2NO2
2NO2 + 2NaOH ® NaNO3 + NaNO2 + H2O
Oxygen if any is removed by introducing alkaline
pyrogallol in the globe.
The supply of air and electric discharge is shut after
some time and the remaining mixture of
noble gases is pumped out.
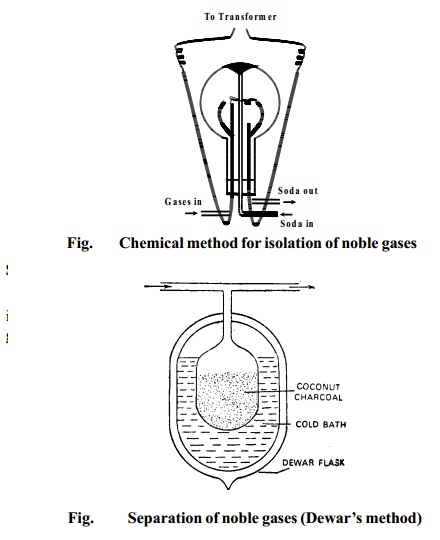
Step 2 Separation of noble gases (DEWAR'S METHOD)
The mixture of noble gases obtained by the above method
is separated into individual
constituents by the use of coconut charcoal which adsorbs different gases at different temperatures.
The mixture of noble gases is passed into a
double-walled bulb containing coconut
charcoal and placed in a low temperature bath at 173K. It is allowed to remain in contact with the charcoal for about half an
hour. At 173K, only argon,
krypton and xenon are adsorbed by the charcoal while
helium and neon remain unadsorbed. These
are pumped out and collected.
The mixture of helium and neon is kept in contact with
coconut charcoal at 93K which completely
adsorbs neon leaving free helium.
The charcoal at 173K containing argon, krypton and xenon
is placed in contact with another charcoal at the temperature of the
liquid air when argon diffuse into the other charcoal.
The temperature of the first charcoal (temp.173K) still containing krypton and xenon is raised to 183K when krypton is set free while xenon remain adsorbed in the charcoal. When it is heated, xenon is recovered.
Related Topics