Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Heavy water: Preparation, Principle, Properties, Important reactions, Uses
Heavy water
It is also called as deuterium oxide. The oxide
of heavy hydrogen (deuterium) is called heavy water. Heavy water was discovered
by Urey in 1932. By experimental data he showed that `ordinary water', H2O
contains small proportion of heavy water, D2O (about 1 part in 5000).
Preparation: The main source of heavy water is the ordinary water from which it is isolated. Generally it is prepared by exhaustive
electolysis.
Principle: The
heavy water is isolated either by prolonged electrolysis or by fractional distillation of water containing alkali. Taylor,
Eyring and First in 1933 formulated the electrolysis of water in seven stages
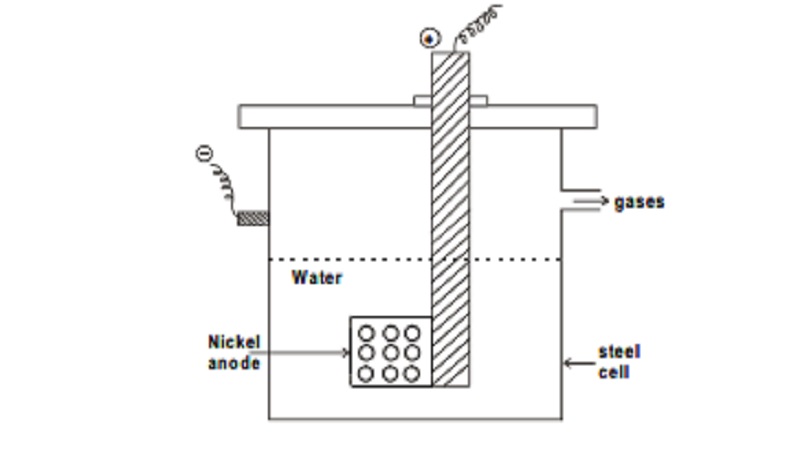
using N/2-NaOH solution and strip nickel electrodes.
The cell consists of a steel cell 18 inches long and 4 inches in
diameter. The cell itself serves as the cathode while the anode consists of a
cylindrical sheet of nickel with a number of holes punched in it. A large
number of such cells are used for electrolysis of water in several stages. The
gases obtained from each stage are separately burnt and the water thus formed
is returned to the previous stage. The heavy water gradually concentrates in
the residue left behind. The process usually consists of five stages.
A partial separation of heavy water from
ordinary water can be affected by fractional distillation. This method utilises
the small difference in boiling points of protium oxide (H2O) and deuterium
oxide (D2O).
Comparison of water
and heavy water
Property : H2O - D2O
Density at : 20oC - 0.998 1.017
Freezing point : 0oC - 3.82oC
Boiling point : 100oC 101.42oC
Maximum density : 1.000 (4oC) - 1.1073
(11.6oC)
Specific heat at : 20oC : 1.00 - 1.01
Surface tension at 20oC : 72.8 dynes/cm - 67.8 dynes/cm
Dielectric constant : 82.0 - 80.5
Viscosity at 20oC : 10.09 millipoises - 12.6
millipoises
The solubilities of substances in heavy water
also differ from those in ordinary water. Thus sodium chloride is about 15%
less soluble in heavy water than in ordinary water.
Physical Properties
Heavy water is a colourless, odourless and
tasteless mobile liquid. Higher viscosity of heavy water is responsible for
lower solubility of ionic solids like NaCl and smaller mobilities of ions.
Chemical Properties
The difference in chemical behaviour between H2O and D2O is very slight.
However, the reaction velocity in general is slightly less in case of D2O
reactions.
Important reactions of
heavy water
1.With metals
D2O reacts slowly with alkali and
alkaline earth metals liberating heavy hydrogen
2 Na + 2 D2O -- > 2 NaOD + D2
(Sodium deuteroxide)
Ca + 2 D2O -- > Ca (OD)2 + D2 (Calcium
deuteroxide)
2. With metallic oxides
Metals like sodium and calcium dissolve in D2O and form heavy
alkalies.
Na2O + D2O ® 2 NaOD
CaO + D2O ® Ca(OD)2
3. With
acid anhydrides
D2O forms corresponding acids containing heavy hydrogen.
SO3 + D2O -- > D2 SO4
Deutero sulphuric acid
P2O5 + 3D2O -- > 2D3 PO4
Deuterophosphoric acid
4. Upon electrolysis, heavy water containing dissolved P2O5,
decomposes into deuterium and oxygen which are liberated at the cathode and
anode respectively.
2D2O à 2D2 + O2
5. With salt and other compounds they form deuterates.
Cu SO4. 5 D2O, Na2 SO4. 10D2O, NiCl2.6D2O
7. Exchange reactions
When compounds containing hydrogen are treated with D2O,
hydrogen undergoes an exchange for deuterium.
NaOH + D2O ->
NaOD + HOD
NH4 Cl + 4D2O -- > ND4 Cl + 4HOD
Biological Properties
In general heavy water, retards the growth of
living organisms like plants and animals. The tobacco seeds do not grow in
heavy water. Also, pure heavy water kills small fish, tadpoles and mice when
fed upon it. Certain moulds have been found to develop better in heavy water.
Uses of heavy water
1.
As a neutron moderator, in nuclear reactors.
2.
It is used as a tracer compound in the study of
reactions occurring in living organisms.
3.
It is used for the preparation of deuterium.
Related Topics