Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Group 2 s - Block Elements
GROUP 2 s
- BLOCK ELEMENTS
The second group of the periodic table contains Beryllium (Be), Magnesium (Mg), Calcium (Ca),
Strontium (Sr), Barium (Ba) and Radium (Ra). These elements are also a known as
"Alkaline Earth Metals". The word earth was applied in old days to a
metallic oxide and because the oxides of calcium, strontium and barium produced
alkaline solutions in water and, therefore these metals are called the alkaline
earth metals. Radium corresponds to all the alkaline earth metals in its
chemical properties but being radioactive, it is studied along with other
radioactive elements.
Like the alkali metals, they are very reactive and hence never occur in
nature in free form and react readily with many non metals.
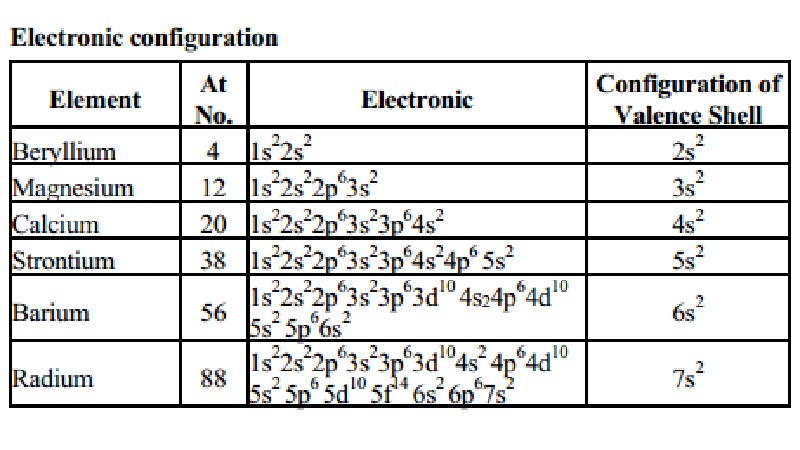
Electronic
configuration
Element At Electronic Configuration
of
No. Valence Shell
Beryllium (4) : 1s22s2 - 2s2
Magnesium (12) : 1s22s22p63s2 - 3s2
Calcium (20) : 1s22s22p63s23p64s2 - 4s2
Strontium (38) : 1s22s22p63s23p64s24p6 5s2 - 5s2
1s22s22p63s23p63d10
4s24p64d10 - 2
Barium (56) : 5s2 5p66s2 6s
1s22s22p63s23p63d104s2
4p64d10 - 2
Radium (88) : 5s2 5p6 5d10 5f14 6s2 6p67s2 - 7s
The electronic configurations show that for each element, the neutral
atom has two electron after inert gas core and two electrons are in a completed
s-subshell. Thus, the outer electronic configuration of each element is ns2
where n is the number of the valence shell. It can be expected that the two
electrons can be easily removed to give the inert gas electronic configuration.
Hence these elements are all bivalent and tend to form ionic salts. Thus ionic
salts are less basic than group 1. Due to their alike electronic structure,
these elements resemble closely in physical and chemical properties.
The variation in physical properties are not as
regular as for the alkalimetals because the elements of this group do not
crystallise with the same type of metallic lattice.
These elements have been sufficiently soft yet
less than the alkalimetals as metallic bonding in these elements has been
stronger than in first group alkali elements.
Beryllium is unfamiliar, partly because it is not very abundant and
partly because it is difficult to extract. Magnesium and calcium are abundant
and among the eight most common elements in the earth's curst. Strontium and
barium are less abundant but are well known, while radium is extremely scarce and
its radioactivity is more important than its chemistry.
Metallic properties
The alkaline earth metals are harder than the
alkali metals. Hardness decreases, with increase in atomic number. They show
good metallic lustre and high electrical as well as thermal conductivity
because the two s-electrons can easily move through the crystal lattice.
Melting and Boiling Points
Both melting and boiling points do not show
regular trends because atoms adopt different crystal structures. They possess
low melting and boiling points. These are, however, higher than those of alkali
metals because the number of bonding electrons in these elements is twice as
great as group 1 elements.
Atomic radius
The atoms
of these elements are somewhat smaller than the atoms of the corresponding
alkali metals in the same period. This is due to higher nuclear charge of these
atoms which tends to draw the orbital electrons inwards. Due to the smaller
atomic radius, the elements, are harder, have higher melting points and higher
densities than the elements of group 1. Atomic radius is seen to increase on
moving down the group on account of the presence of an extra shell of electron
at each step.
Ionic radius
The ions are also large but smaller than those of the elements in group
1. This is due to the fact that the removal of two orbital electrons in the
formation of bivalent cations M2+, (Be2+, Mg2+,
Ca2+, Sr2+, etc) increases the effective nuclear charge
which pulls the electrons inwards and thus reduces the size of the ions. The
ionic radius is seen to increase on moving down the group 2.
Atomic volume
Due to the addition of an extra shell of
electrons to each element from Be to Ra, the atomic volume increases from Be to
Ra.
Ionisation Energy
As the alkaline earth metals are having smaller size and greater nuclear
charge than the alkali metals, the electrons are more tightly held and hence
the first ionisation energy would be greater than that of the alkali metal.
The second ionisation energy has been to be nearly double than that of
the first ionisation energy.
It is interesting to observe that although the IE2 of the alkaline earth
metals is much higher than the IE1 they are able to form, M2+
ions. This is due to their high heat of hydration in aqueous solution and high
lattice energy in the solid state. As the atomic size gets increased from Be to
Ba, the values of IE1 and IE2 of these elements would
decrease on going down the group, ie, Be to Ba.
As among second group elements beryllium has the highest ionisation energy.
It has the least tendency to form Be2+ ion.
Thus its compounds with nitrogen, oxygen,
sulphur and halogens are covalent whereas the corresponding compounds of Mg,
Ca, Sr and Ba are ionic.
The total energy required to produce gaseous divalent ion for second
group elements is over four times greater than the amount needed to ionise
alkali metals. This very high energy requirement is more than offset by the
hydration energy or the lattice energy being more than four times greater.
Oxidation states
Because of the presence of two s-electrons in the outermost orbital,
being high heat of hydration of the dipositive ions and comparatively low value
of IE2, the alkaline earth metals have been bivalent. The divalent ion is
having no unpaired electron, hence their compounds are diamagnetic and
colourless, provided their anions have been also colourless.
Flame colouration
These elements and their compounds impart characteristic colours to
flame. Thus, barium - apple green, calcium - brick red, strontium - crimson
red, radium - crimson red.
The reason for imparting the colour to flame is that when elements or
their compounds are put into flame, the electrons get energy and excite to
higher energy levels. When they return to the ground state they emit the
absorbed energy in the form of radiations having particular wavelength.
Beryllium and magnesium atoms are smaller and their electrons being
strongly bound to the nucleus are not excited to higher energy levels.
Therefore they do not give the flame test.
Diagonal relationship between Beryllium and
Aluminium
In case of beryllium, a member of second period
of the periodic table, which resembles more with Aluminium group (13 group)
than the member of its own group (2nd). The anamolous behaviour of beryllium is
mainly ascribed to its very small size and partly due to its high
electronegativity. These two factors tend to increase the polarising power of
Be2+ tends to form ions to such extent that it is significantly
equal to the polarising power of Al3+ ions. Thus the two elements
resemble very much.
Related Topics