Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Group - 14 Elements - The Carbon Family
GROUP - 14 ELEMENTS - THE CARBON FAMILY
The group 14 (IVA) elements - carbon, silicon, germanium,
tin and lead are especially
important both in industry and in living organisms.
1.
Carbon is an
essential constituent of the molecules on which life is based.
2.
Silicon is the
second most abundant element in the earth's crust.
3.
Bothsilicon and
germaniumare used in making modern solid-state electronic devices.
4.
Tin and lead have
been known and used since ancient times.
General Trends
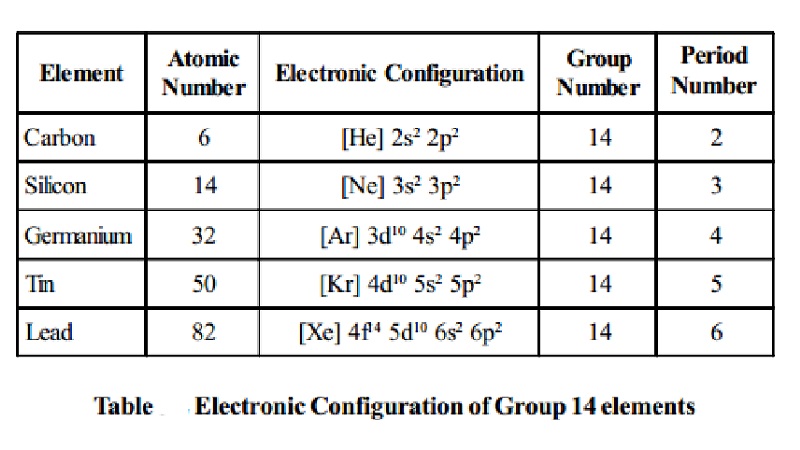
Electronic configuration: The elements of this group possess ns2 np2 electronic
configuration.
Electronic Configuration of Group 14 elements
Carbon -
Atomic Number :
6 Electronic Configuration
: [He]
2s2 2p2 Group Number : 14
Period Number : 2
Silicon -
Atomic Number :
14 Electronic Configuration : [Ne]
3s2 3p2 Group Number : 14
Period Number : 3
Germanium -
Atomic Number :
32 Electronic Configuration
: [Ar]
3d10 4s2 4p2 Group
Number : 14 Period Number : 4
Tin -
Atomic Number : 50
Electronic Configuration : [Kr] 4d10 5s2 5p2 Group Number
: 14 Period Number : 5
Lead -
Atomic Number :
82 Electronic Configuration
: [Xe]
4f14 5d10 6s2 6p2 Group Number
: 14 Period Number : 6
1 Silicones - structure and uses
The silicones are a group of organosilicon polymers.
They have a wide variety of
commercial uses.
The complete hydrolysis of SiCl4 yields silica SiO2, which has a very stable three-dimensional structure. The fundamental research of
F.S. Kipping on the hydrolysis of
alkyl-substituted chlorosilanes led, not to the expected silicon compound analogous to a ketone, but to long-chain
polymers called silicones.
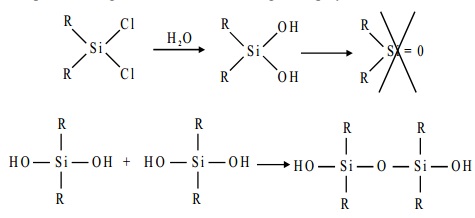
The starting materials for the manufacture of silicones
are alkyl-substituted chlorosilanes. Thus
the hydrolysis of trialkylmonochlorosilane R3SiCl yields hexa- alkylsiloxane.
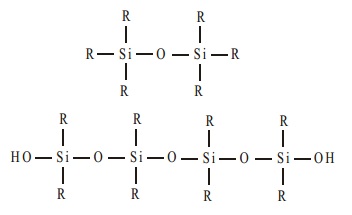
The dialkyldichlorosilane R2SiCl2 on hydrolysis gives rise to straight chain polymers
and, since an active OH group is left at each end of the chain, polymerisation continues and the chain increases
in length.
The hydrolysis of alkyl tricholorosilane RSiCl3 gives a very complex cross- linked polymer.
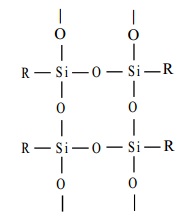
Uses
1.
Silicones act as
excellent insulators for electric motors and other appliances as they can
withstand high temperatures.
2.
Straight chain
polymers of 20 to 500 units are used as silicone fluids. They are water repellent because of the organic side group.
These polymers are used in
waterproofing textiles, as lubricants and as polish.
3.
Silicone rubber
retain their elasticity even at low temperatures and resist chemical attack. They are mixed with paints to make them
damp-resistant.
4.
Silicone resins, a
cross-linked polymer used as non-stick coating for pans and are used in paints and varnish.
5.
Silicone oils are
highly stable and non-volatile even on heating. Hence used for high temperature oil bath, high vacuum pump etc.
2
Metallurgy of Lead
Ores
1. Galena
PbS
2. Cerrusite
PbCO3
3. Anglesite PbSO4
4. Lead
ochre PbO
Extraction:
Lead is mainly extracted from the sulphide ore galena. Galena
contains lead sulphide and small quantities of silver.
1. Concentration: The ore is concentrated by froth floatation process.
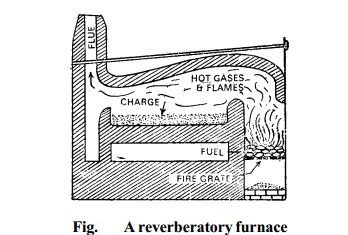
2. Smelting in a Reverberatory furnace: The concentrated ore is roasted in a reverberatory furnace at a moderate temperature.
The temperature of furnace is controlled
by regulating the air supply. During roasting, galena is partly oxidized to lead monoxide and partly to lead sulphate.
2PbS + 3O2 ® 2 PbO +
2SO2
PbS + 2O2 ® PbSO4
More of galena is then added. The temperature is raised and simultaneously the air supply is reduced. Lead sulphide reacts with the two oxidised products giving lead.
PbS+2PbO ® 3Pb+SO2
PbS+PbSO4 ® 2Pb+2SO2
Thus in this process roasting and smelting are carried
out in the same furnace,
at two different temperatures.
About 90% of lead is obtained as metal, the rest passes
into slag. Lead is
recovered from the slag by heating with lime and
powdered coke.
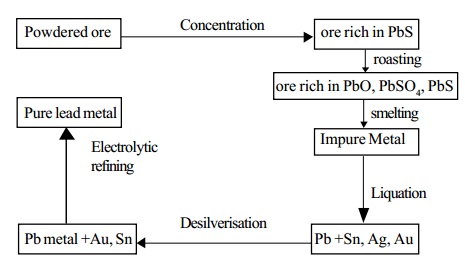
Purification of Lead
Lead extracted by the above method contains impurities such as silver, copper, tin, bismuth, gold and iron. It is refined by the following processes.
a. Liquation
The impure metal is heated on a sloping hearth. Lead melts and flows down the slope. The infusible impurities remain on the hearth.
b. Desilverisation
Silver is removed by either Pattinson's process or
Park's process.
c. Electrolytic refining
Very pure lead is obtained by this process.
Anode - Impure lead
Cathode - Very pure lead
Electrolyte - Lead fluosilicate + Hydrofluosilicic Acid
(PbSiF6) (H2SiF6)
The metallic impurities which are more electropositive
than lead, such as iron and tin, go into the solution while the rest of the
impurities are thrown down as anode mud.
Physical
properties
1.
Lead is a bluish
grey metal with a bright luster.
2.
It is soft and can
be cut with a knife and drawn into a wire and rolled into a sheet.
3.
It is not a good
conductor of heat and electricity. It marks paper.
Chemical properties
1. Action of air
i.
It is unaffected by
dry air but in moist air a layer of lead carbonate or lead hydroxide is deposited on its surface which protects it
from further action of air.
ii.
When heated in air
or oxygen, lead is oxidized to litharge (PbO) and red
lead (Pb3O4)
2Pb
+ O2 ® 2PbO 3Pb + 2O2 ®
Pb3O4
2. Action of water
Lead is not attacked by pure water in the absence of air,
but water containing dissolved air has
a solvent action on it due to the formation of lead hydroxide (a poisonous
substance). This phenomenon is called Plumbo solvency.
2Pb + O2 + 2H2O ® 2Pb(OH)2
3. Action of acids
i) Dilute H2SO4 and HCl
have no action on lead.
ii) Hot Conc. H2SO4 liberates
SO2 but the reaction is retarded by the formation
of an insoluble layer of lead sulphate.
Pb + 2H2SO4® PbSO4 + 2H2O + SO2
iii) Concentrated HCl evolves hydrogen and also forms
Chloroplumbic acid
Pb + 2HCl ® PbCl2 +H2
PbCl2 + 2HCl --- > < --- H2PbCl4 (chloroplumbic acid)
Uses: Lead is
used
i.
For making lead
pipes,
ii.
For making
telegraph and telephone wires,
iii.
In making bullets
and lead accumulators,
iv.
In lead chambers,
for the manufacture of sulphuric acid,
v.
For making alloys
like solder, pewter and type metal,
vi.
For preparing
tetraethyl lead (Pb(C2H5)4) which is
used as an additive to petrol to prevent
knocking
Problem
An element A belongs to 14th group and occupies period number 6. A
reacts with conc. HCl. to give B an acid. A
is used to prepare C which is used as an antiknock in automobiles.
Identify the element A and the compounds B and C Write the reactions.
Solution
1. As per the position in the periodic table, the
element A is lead. 2. Lead with
Conc. HCl gives B
Pb + 4 HCl ® H2PbCl4 + H2
∴ Compound B is chloroplumbic acid.
3. Compound C is tetraethyl lead.
Related Topics