Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
General Properties of Actinide Series
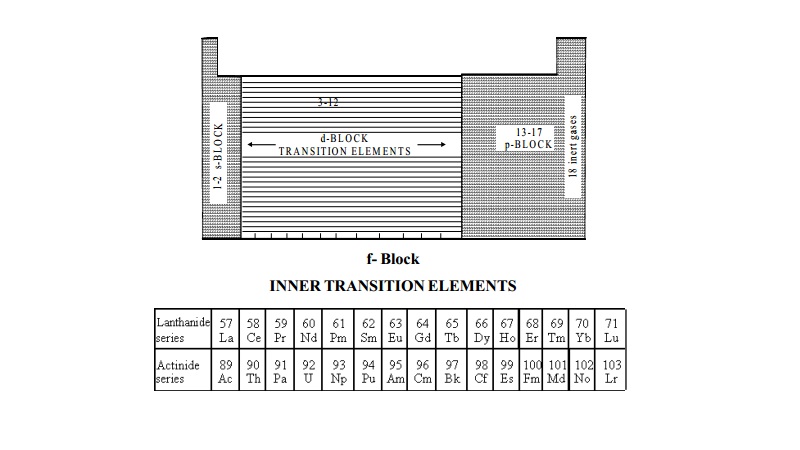
The position of f block elements in the periodic table, is explained
above.
The elements in which the extra electron enters ( n- 2 )f
orbitals are called f- block elements.
These elements are also called as inner transition elements because they form a transition series within the
transition elements. The f-block elements
are also known as rare earth elements. These are divided into two series.
i) The Lanthanide series (4f-block elements)
ii) The Actinide series (5f- block elements )
The Actinide Series (5f block elements)
In 1923 Neils Bohr postulated the existence of an
actinide series analogous to the lanthanide series.
The fifteen elements from actinium to lawrencium constitute
the actinide series of the periodic table.
General Properties of Actinide Series
1. The general electronic configuration of actinides is [Rn]
5f0,1-14 6d0,1-2 7s2 where
Rn stands for radon core.
2. Oxidation states
These elements shows the oxidation states of +2, +3, +4,
+5 and +6. Out of these, +4
oxidation state is most common state.
3. Radii of M3+ and M4+ ions
The ionic radii of actinide elements decrease gradually
as we move along the actinide
series. The steady decrease in the ionic radii with increase in nuclear charge is called actinide contraction and is analogous
to lanthanide contraction.
Cause of actinide contraction
Cause of actinide contraction is the imperfect shielding
by 5f-electrons. As we proceed from one
element to the next one in actinide series, the nuclear charge increases by +1 at each next element which is not
compensated due to poor shielding
effect of 5f orbitals due to their more diffuse shape. Hence as the atomic
number increases, the inward pull experienced by 5f-electrons increase. Consequently steady decrease in size occurs in the
actinide series.
Related Topics