Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Fajan's rules: Covalent character of ionic bonds
Fajan's rules
Covalent character of
ionic bonds
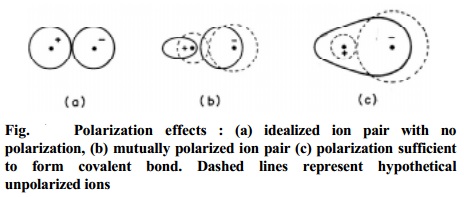
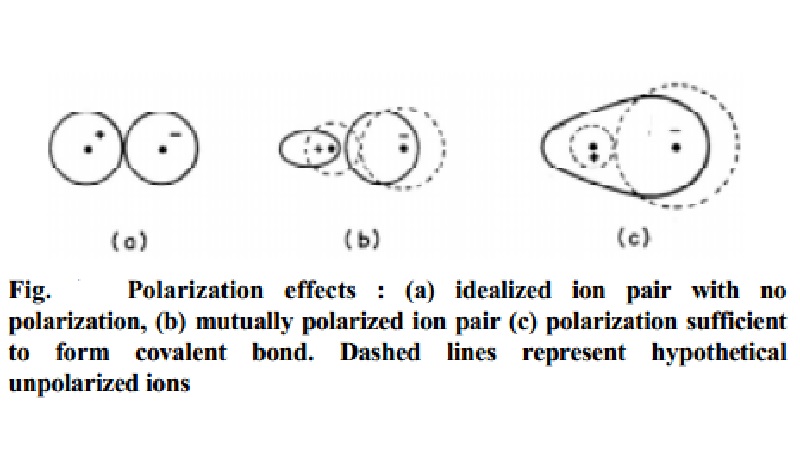
Fig. shows
Polarization effects : (a) idealized ion pair with no polarization, (b)
mutually polarized ion pair (c) polarization sufficient to form covalent bond.
Dashed lines represent hypothetical unpolarized ions
When cations and anions approach each other, the
valence shell of anions are pulled towards cation nucleus due to the coulombic
attraction and thus shape of the anion is deformed. This phenomenon of
deformation of anion by a cation is known as polarization and the ability of cation to polarize a nearby anion
is called as polarizing power of cation.
Fajan points out that greater is the
polarization of anion in a molecule, more is covalent character in it. This is Fajan's rule.
Fajan also pointed out the influence of various factors on cations for
polarization of anion.
1.
When the size of a cation is smaller than a
cation with the same charge, then the smaller sized cation causes a greater
extent of polarization on the anion than the larger sized cation.
2.
The polarizing capacity of a cation is related
to its ionic potential (which is Z+/r) which is inversely related to
the ionic radius. Therefore comparing Li+ and Na+ or K+
ions, although these cations have single positive charge, Li+ ion
polarizes an anion more than Na+ or K+ ions can do on the
same anion. This is because of the smaller size of Li+ than Na+
or K+ ions.
3.
Greater the polarization effects greater will be
the covalent character imparted into the ionic bond.
The general trend in the polarizing power of
cations: Li+ > Na+ > K+ > Rb+
> Cs+
covalent character:
LiCl > NaCl > KCl > RbCl > CsCl.
a) Size of the anion
When the size of anion is larger, valence electrons are less tightly
held by its nucleus. Therefore more effectively the cation pulls the valence
electrons towards its nucleus. This results in more polarization effect. That
is, for the same charge of the anion, larger sized anion is more polarized than
a smaller sized anion.
The trend in the polarization of anions:
I- > Br- > Cl-
> F-
\ covalent character :
LiF < LiCl < LiBr < LiI
b) Charge on cation
If the oxidation state of the cation is higher the polarization of anion
will be more. Thus more will be covalent nature in the bonding of the molecule.
Thus polarizing power: Fe+2 < Fe+3
Covalent character : FeCl2 < FeCl3
c) Presence of polar
medium
Presence of a polar medium keeps away the cations and anions from each
other due to solvation. This prevents polarization of anion by the cation.
Therefore AlCl3 behaves as an ionic molecule in water, while it is a
covalent molecule in the free state.
Related Topics