Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Electrical Conductance Quantities
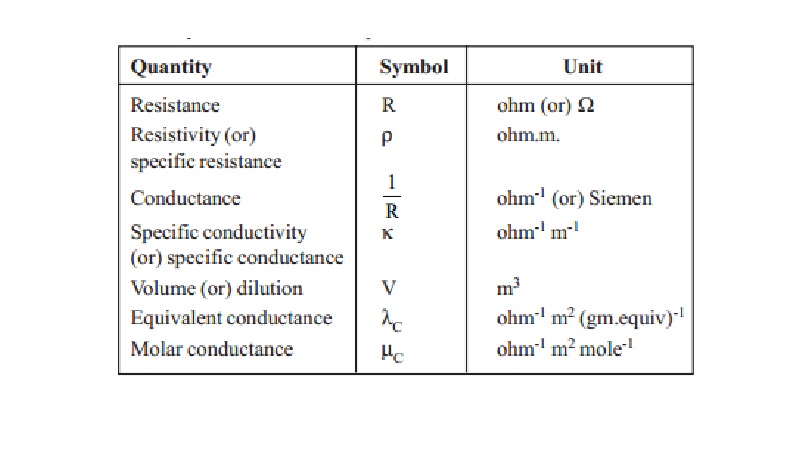
ELECTRICAL CONDUCTANCE QUANTITIES
The S.I. unit of electrical current is ampere.
The unit of quantity of electricity is coulomb. when one ampere of current is
passed for one second, then the quantity of current passed is one coulomb.
(i.e.,) Q = I x t
Coulomb
Ohm's law : This law can be stated as, at constant
temperature, the strength of the
current flowing through a conductor is directly proportional to the potential
difference and inversely proportional to the resistance of the conductor.
Thus, I = V / R
V = IR
V = Volts, I = ampere, R = ohms
Specific resistance : The resistance 'R' ohms offered by the material
of the conductor to the flow of
current through it is directly proportional to its length (l) and inversely proportional to the area of cross section (a). Thus,
R
a l/a and
R
= ρ (l/a)
ρ is
called the specific resistance and it is resistance in ohms which one meter
cube of material offers to the passage of electricitiy through it, unit of
specific resistance is ohm-meter.
Specific conductance : The reciprocal of specific resistance is called
as specific conductance (or)
specific conductivity (k) [k is called 'kappa'].
k is
defined as the conductance of one metre cube of an electrolyte solution
k = 1/ ρ = 1/R . l/a
Unit of specific conductance is ohm-1
m-1 (or) mho.m-1
Since ohm-1 = mho
K
= 1/ohm x m/m2 = ohm-1m-1
Also, 1 siemen = 1 mho. \ k is also
expressed as S.m-1.
Conductance is the reciprocal of resistance 'R'.
Conductance = 1/R
( l/a ) is called as the cell constant (m-1)
and is constant for a given conductance cell.
Thus specific conductance 'k' = cell
constant x
conductance = Cell constant / Resistance
Equivalent conductance : Equivalent conductance (lC) is defined as the conductance of an electrolyte solution containing one gram
equivalent of the electrolyte. It is equal to the product of specific
conductance (k) of the
solution and the volume (V) of the solution that contains one gram equivalent
of the electrolyte.
(lC) = k x V
In general if an electrolyte solution contains
N gram-equivalents in 1,000 cc of the solution the volume of the solution
containing 1 gram equivalent will be
1000/N x 10-6m3 (1 cc= 10-6m3 )
lC = k 10-3
/ N mho.m2.(gm.equiv)-1
for 1 : 1 electrolyte normality N equals to
molarity 'C'. Then
lC = k 10-3
/ N mho.m2.(gm.equiv)-1
lC values
depend on the type of the electrolyte, concentration of the solution
and temperature.
Molar conductance : Molar conductance ' mC' is defined as the conductance of a solution containing one mole of the electrolyte
dissolved in it.
= (k x 10-3 ) / C mho.m2.mole-1
where M is the molarity of the electrolyte
solution.
For 1 : 1 electrolyte like NaCl, equivalent
conductance is equal to molar conductance.
Related Topics