Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Dual Property of an Electron
DUAL PROPERTY OF AN
ELECTRON
In case of light, some phenomena like
interference, diffraction etc., can be explained if light is supposed to have
wave character. However certain other phenomena such as black body radiation
and photo electric effect can be explained only if it is believed to be a
stream of photons i.e., has particle character. Thus light is said to have a
dual character. Such studies on light were made by Einstein in 1905.
Louis de Broglie, a French Physicist, in 1924,
advanced the idea that like photons, all material particles such as electron,
proton, atom, molecule, a piece of chalk, a piece of stone or iron ball
possessed both wave character as well as particle character. The wave
associated with a particle is called a matter wave.
Difference between a
particle and a wave
The concept of a particle and a wave can be
understood by the different points of distinction between them.
PARTICLE
1. A particle occupies a
well-defined position in space i.e a
particle is localized in space e.g. a
grain of sand, a cricket ball etc.
2. When a particular space is occupied
by one particle, the same space cannot be occupied simultaneously by any other
particle. In other words, particles do not interfere.
3. When a number of particles are
present in a given region of space, their total value is equal to their sum i.e
it is neither less nor more.
WAVE
1. a wave is spread out in space e.g. on
throwing a stone in a pond of water, the waves start moving out in the form of
concentric circles. Similarly, the sound of the speaker reaches everybody in
the audience. Thus a wave is delocalized in space.
2. Two or more waves can coexist in the
same region of space and hence interfere.
3. When a number of waves are present in
a given region of space, due to interference, the resultant wave can be larger
or smaller than the individual waves i.e. interference may be constructive or
destructive.
Experiments to prove
particle and wave property of Electrons
a)
Verification of Wave character
i)
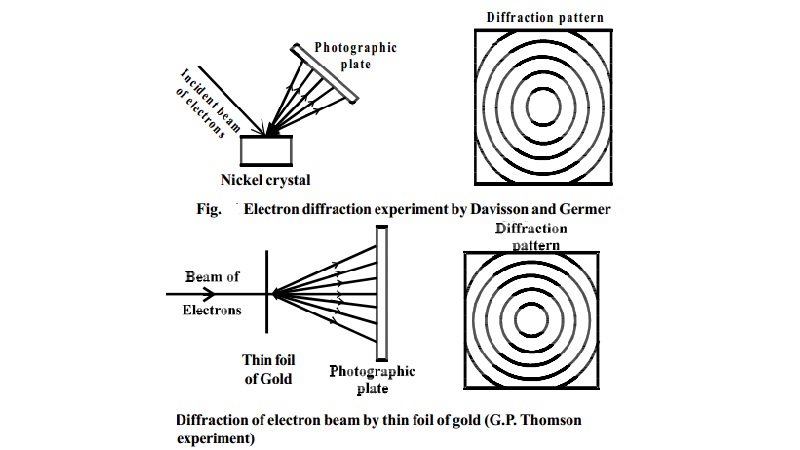
Davisson and Germer's Experiment
In 1927 Davisson and Germer observed that, a beam of electrons obtained
from a heated tungsten filament is accelerated by using a high positive
potential. When this fine beam of accelerated electron is allowed to fall on a
large single crystal of nickel, the electrons are scattered from the crystal in
different
directions.
The diffraction pattern so obtained is similar to the diffraction pattern
obtained by Bragg's experiment on diffraction of X-rays from a target in the
same way (Fig.).
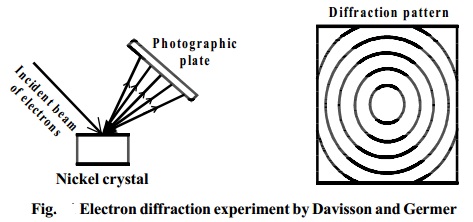
diffraction experiment
by Davisson and Germer
Since X-rays have wave character, therefore, the electrons must also
have wave character associated with them. Moreover, the wave length of the
electrons as determined by the diffraction experiments were found to be in
agreement with the values calculated from de-Broglie equation.
From the above discussion, it is clear that an electron behaves as a
wave.
ii)
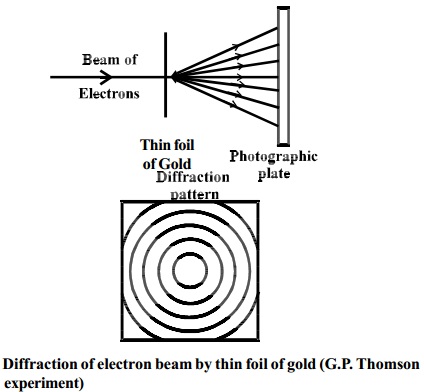
Thomson's experiment
G.P.
Thomson in 1928 performed experiments with thin foil of gold in place of nickel
crystal. He observed that if the beam of electrons after passing through the
thin foil of gold is received on the photographic plate placed perpendicular to
the direction of the beam, a diffraction pattern is observed as before (Fig.).
This again confirmed the wave nature of electrons.
b)
Verification of the particle character
The particle character of the electron is proved by the following
different experiments:-
i)
When an electron strikes a zinc sulphide screen,
a spot of light known as scintillation is produced. A scintillation is
localized on the zinc sulphide screen. Therefore the striking electron which
produces it, also must be localized and is not spread out on the screen. But
the localized character is possessed by particles. Hence electron has particle
character.
ii)
Experiments such as J.J.Thomson's experiment for
determination of the ratio of charge to mass (i.e. e/m) and Milliken oil drop
experiment for determination of charge on electron also show that electron has
particle character.
iii)
The phenomenon of Black body radiation and
Photoelectric effect also prove the particle nature of radiation.
Related Topics