Chapter: Biochemical Pharmacology : Drugs that act on sodium and potassium channels
Calcium in muscle cell function
Calcium in muscle cell
function
Calcium has a pivotal role in
the control of muscle cell action. Muscle cells occur in different types:
• Smooth muscle cells. They occur in hollow
organs, including blood vessels,
• Heart muscle cells,
• Skeletal muscle cells.
Heart and skeletal muscle
together are classified as striated muscle yet do have some important
functional differences (see below). The muscle cells in vessel walls control
the blood pressure, which makes them important drug targets; that heart muscle
cells are important targets, too should go without saying.
We will first look at the
role of calcium in the contraction of striated muscle. Figure 6.2a shows a
light-microscopic picture of heart muscle. The striations are oriented
perpen-dicularly to the longitudinal axis of the cells. The borders between the
individual heart muscle cells are bridged by gap junctions, which will ensure
swift spread of excitation from one cell to the next. Skeletal muscle cells
form long syncytia in which the excitation spreads even faster.
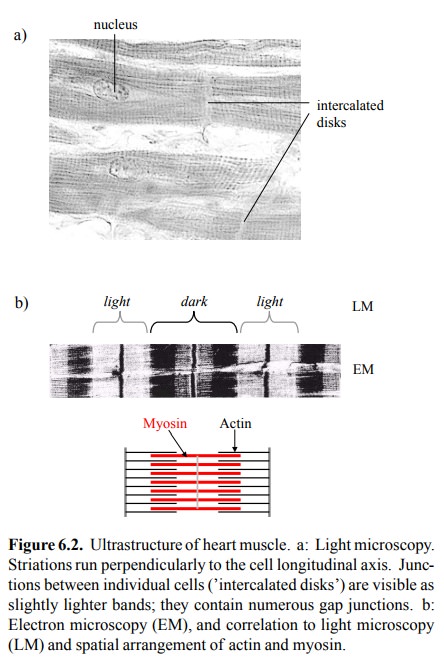
At electron microscopic
resolution, the striations appear more complex (Figure 6.2b). They correspond
to densely and regularly packed filaments of actin and myosin, each composed of
numerous, linearly polymerized subunits2. The finer striations
visible in EM are due in part to addi-tional structural proteins, and in part
to zones of overlap be-tween actin and myosin.
While actin and myosin are
present and responsible for motility in essentially all cells, a peculiarity of
the striated muscle (apart from the sheer amount and regular, parallel packing)
is the presence of two additional proteins associ-ated with the actin
filaments. These proteins are troponin and tropomyosin, and they are crucial in
the control of con-traction.
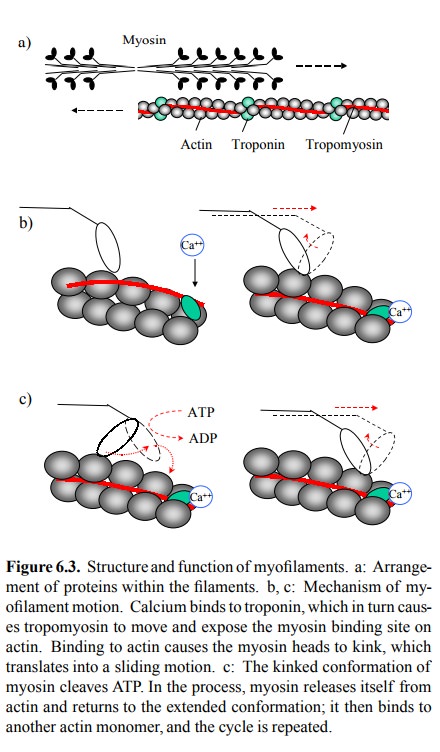
The arrangement and the
workings of actin, myosin, tro-ponin and tropomyosin in striated muscle are
summarized in Figure 6.3. The myosin heads are in close proximity to the actin
filaments, but in the resting state direct contact be-tween actin and myosin is
blocked by the tropomyosin fil-aments. Upon cell excitation, calcium becomes
available and binds to troponin, which in turn moves the tropomyosin out of the
way. The heads of myosin are allowed to access their binding sites on actin.
Binding causes the myosin head to bend. This will both propel the myosin
filament along the actin `track'and trigger the intrinsic ATPase activ-ity of
myosin. The energy of ATP cleavage is used to pow-er cycles of binding, bending
and release. This activity is commonly likened to rowing; the myosin heads,
then, com-prise both the oar and the biceps, whereas the actin filament is
merely the water. Interestingly, the ATP is actually ex pended at the stage of
the `push' rather than `pull' – which is where the analogy ends.
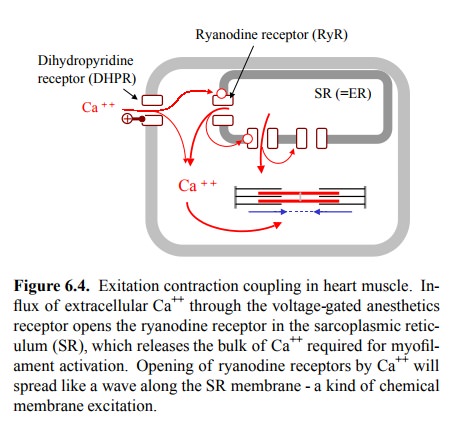
In striated muscle, the sheer
amount of filaments is such that we actually need quite a bit of calcium to
swiftly sat-urate the troponin molecules and trigger contraction. The lion's
share of this calcium is not obtained from the extra-cellular space (via the
voltage-gated Ca++ channel, the dihy-dropyridine receptor – see
later) but from the intracellular storage, more specifically from the
endoplasmic reticulum, which somebody found necessary to christen
`sarcoplas-mic' reticulum in the muscle cell (gr. sarx, sarkos = flesh). It is
released from there by a specialized Ca++ channel, the ryanodine
receptor (RyR)3. This channel is activated by cy tosolic calcium, which of
course creates a fast and powerful amplification mechanism for the release of
calcium from the ER and, thus, for contraction (Figure 6.4).
The anatomical arrangement of
the cell membrane, the SR, and the myofilaments in the striated muscle is
further opti-mized for rapid action. Figure 6.5 illustrates this for a skele-tal
muscle cell. The cytoplasmic membrane there forms in-vaginations called T
(transversal) tubules, which protrude into the cell interior and enter into
immediate contact with the SR cisterns, which in turn are aligned to the
contractile filaments.
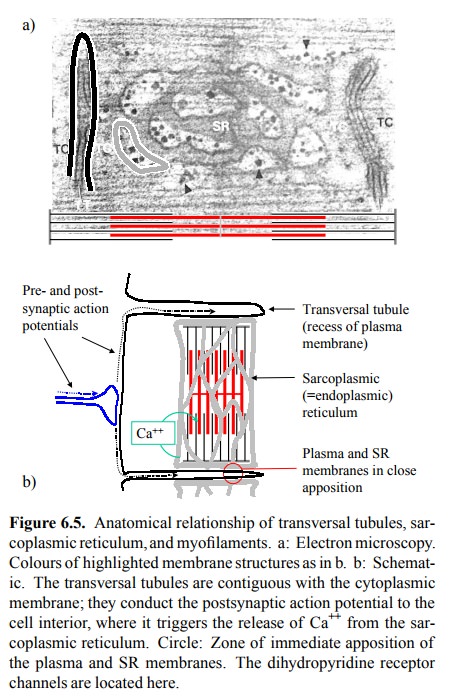
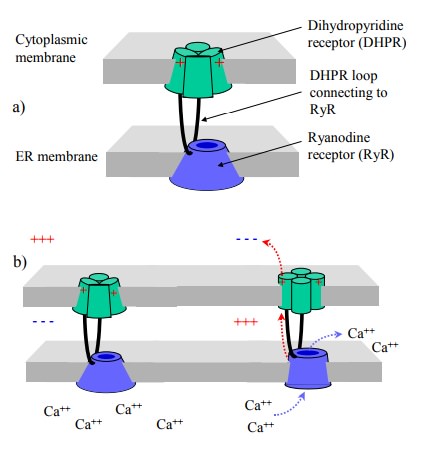
The close alignment of
cytoplasmic and ER membranes is, in fact, crucially important for the workings
of excitation-contraction coupling in the skeletal muscle. In these cells, we
have a unique mechanism of activation of one channel by another: The RyR is directly
hooked up to a cytosolic loop of the dihydropyridine receptor (DHPR; Figure
6.6a, b). Membrane depolarization will cause a conformational change to the
DHPR, which in turn is directly and mechan-ically transmitted to the RyR, so
that both channels open synchronously. This even works in the absence of any
cal-cium flux across the cytoplasmic membrane – experimen-tally, skeletal
muscle cells can be induced to contract in calcium-free buffers.
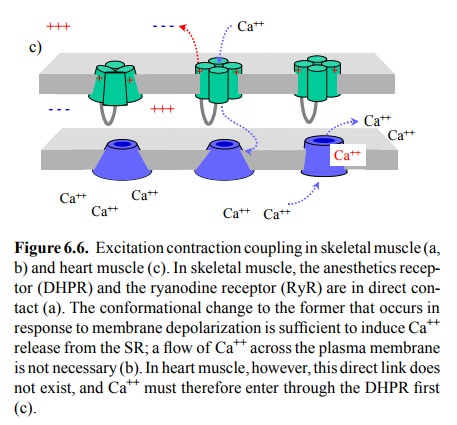
Although the anatomical
relationships are closely similar in the heart muscle, there is no direct
coupling between the DHPR and the RyR in this case (Figure 6.6c). There-fore,
excitation-contraction coupling in the heart muscle cells does require flux of calcium across the DHPR channel, even if only
as a trigger, which will then activate the RyR and release the lion's share of
the calcium needed for acti-vating troponin from the SR. Thus, drugs that
reduce flow of Ca++ through the DHPR channel will reduce the force
of contraction in the heart (and in smooth muscle, see later) but not the
skeletal muscle.
Related Topics