Chapter: 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Brief Questions and Answers: Chemistry Periodic Classification of Elements
Periodic Classification of Elements | Chemistry
Answer the following questions
24. Define modern periodic law.
The
modern periodic law was developed which states that "the physical and
chemical properties of the elements are periodic functions of their atomic
numbers".
25. What are isoelectronic ions? Give examples.
Ions
with same number of electrons are called isoelectronic ions. Eg.Na+,
F, Mg2+, Ne(10eŌłÆ), Ar,ClŌłÆ, K+(18eŌłÆ).
26. What is effective nuclear charge ?
The
net nuclear charge experienced by valence electrons in the outermost shell is
called the effective nuclear charge. It is approximated by the below mentioned
equation.
Zeff
= Z-S Here, Z - atomic number,
S
- Screening constant.
27. Is the definition given below for ionisation enthalpy is correct?
"Ionisation enthalpy is defined as the energy required to remove the most loosely bound electron from the valence shell of an atom"
Correct.
Ionisation enthalpy is defined as the minimum amount of energy required to
remove the most loosely bound electron from the valence shell of the isolated
neutral gaseous atom in its ground state.
M(g)+
IE1 ŌåÆ M+(g)+ 1eŌłÆ
Where
IE1 represents the first ionisation energy.
28. Magnesium loses electrons successively to form Mg+, Mg2+ and Mg3+ ions. Which step will have the highest ionisation energy and why?
The
effective nuclear charge of the cation is higher than the corresponding neutral
atom. Thus the successive ionisation energies, always increases in the
following order
Ie1
< IE2 < IE3 < ŌĆ”ŌĆ”ŌĆ”ŌĆ”
Mg2+
has stable inert gas configuration. Removing and electron from it need more
energy.
Thus
Mg2+ + IE3
ŌåÆ
Mg3+ step has highest ionisation enthalpy
29. Define electronegativity.
It
is defined as the relative tendency of an element present in a covalently
bonded molecule, to attract the shared pair of electrons towards itself.
30. How would you explain the fact that the second ionisation potential is always higher than first ionisation potential?
IE2
> IE1. Because the electron must be removed against net positive
charge on the metal ion.
31. Energy of an electron in the ground state of the hydrogen atom is -2.8 x 10-18 J. Calculate the ionisation enthalpy of atomic hydrogen in terms of kJ mol-1.
Energy
of an electron in the ground state of the hydrogen atom (E1) = ŌłÆ2.8 ├Ś
10ŌłÆ18 J
Energy
of an electron at infinity (EŌł×) = 0
the
ionisation enthalpy of atomic hydrogen = EŌł× ŌłÆ E1
= 0 ŌłÆ (ŌłÆ2.8 ├Ś 10ŌłÆ18 J)
= 0 + 2.8├Ś10ŌłÆ18
= 2.8 ├Ś 10ŌłÆ18 ├Ś 10ŌłÆ3 ├Ś
6.023 ├Ś1023 KJ molŌłÆ1
ionisation
enthalpy of atomic hydrogen in terms of kJ molŌłÆ1 = 1.686 ├Ś 103
KJ molŌłÆ1
(or) = 1686 KJ molŌłÆ1
32. The electronic configuration of atom is one of the important factor which affects the value of ionisation potential and electron gain enthalpy. Explain
ŌŚÅ
It is expected that boron(B) has higher ionisation energy than beryllium since
it has higher nuclear charge.
ŌŚÅ
However, the actual ionisation energies of beryllium and boron are 899 and 800
kJ molŌłÆ1 respectively contrary to the expectation.
ŌŚÅ
It is due to the fact that beryllium with completely filled 2s orbital, is more
stable than partially filled valence shell electronic configuration of boron. (2s2,2p1)
ŌŚÅ
In case of elements such as beryllium (1s2,2s2), nitrogen
(1s2, 2s2, 2p3) the addition of extra electron
will disturb their stable electronic configuration and they have almost zero
electron affinity.
ŌŚÅ
Noble gases have stable ns2, np6 configuration, and the
addition of further electron is unfavourable and requires energy. Halogens
having the general electronic configuration of ns2, np5
readily accept an electron to get the stable noble gas electronic configuration
(ns2,np6), and therefore in each period the halogen has
high electron affinity. (High negative values)
33. In what period and group will an element with Z = 118 will be present?
The
electronic configuration is (1s2 2s2 2p6 3s2
3p6 3d10 4s2 4p6 4d10 4f14
5d2 5p6 5d10 5f14 6s2 6p6
6d10 7s2 7p6 ). Therefore, this element
belongs to period No.7 and group No.18 along with inert gases.
34. Justify that the fifth period of the periodic table should have 18 elements on the basis of quantum numbers.
For
5th period, n = 5,
l =0 5s
has 2 electrons = 2 elements
l =1 5p
has 6 electrons = 6 elements
l =2 4d
has 10 electrons = 10 elements
Total
number of orbitals = 1+3+5 = 9
Maximum
no of electrons in 9 orbitals = 9├Ś2 = 18 = 18 elements
35. Elements a, b, c and d have the following electronic configurations:
a: 1s2, 2s2, 2p6
b: 1s2, 2s2, 2p6, 3s2, 3p1
c: 1s2, 2s2, 2p6, 3s2, 3p6
d: 1s2, 2s2, 2p1
which elements among these will
belong to the same group of periodic table
a
and c ŌĆō inert gases ŌĆō 18th group
b
and d ŌĆō boron group ŌĆō 3rd group
36. Give the general electronic configuration of lanthanides and actinides?
Lanthanides
: 4f 1ŌłÆ14 5d0ŌłÆ1 6s2
Actinides
: 5f 0ŌłÆ14 6d0ŌłÆ2 7s2
37. Why halogens act as oxidising agents?
Halogens
having the general electronic configuration of ns2, np5
readily accept an electron to get the stable noble gas electronic configuration
(ns2, np6), and therefore in each period the halogen has
high electron affinity. (high negative values). Hence they act as Oxidising
agents.
38. Mention any two anomalous properties of second period elements.
ŌŚÅ
The elements of the same group show similar physical and chemical properties.
ŌŚÅ
However, the first element of each group differs from other members of the
group in certain properties.
ŌŚÅ
For example, lithium and beryllium form more covalent compounds, unlike the
alkali and alkaline arth metals which predominantly form ionic compounds.
ŌŚÅ
The elements of the second period have only four orbitals (2s & 2p) in the
valence shell and have a maximum co-valence of 4, whereas the other members of
the subsequent periods have more orbitals in their valence shell and shows
higher valences. For example, boron (B) forms BF4 and aluminium
forms A1F63ŌłÆ.
39. Explain the pauling method for the determination of ionic radius.
ŌŚÅ
Ionic radius of uni-univalent crystal can be calculated using Pauling's method
from the inter ionic distance between the nuclei of the cation and anion.
ŌŚÅ
Pauling assumed that ions present in a crystal lattice are perfect spheres, and
they are in contact with each other
therefore,
d = rC++ rA- ŌĆ”ŌĆ”ŌĆ”.
(1)
ŌŚÅ
Where d is the distance between the centre of the nucleus of cation C+
and anion AŌłÆ and rC+ , rAŌłÆ are the radius of the cation
and anion respectively.
Pauling
also assumed that the radius of the ion having noble gas electronic
configuration (Na+ and FŌłÆ having 1s2, 2s2,
2p6 configuration) is inversely proportional to the effective
nuclear charge felt at the periphery of the ion.
i.e.
rC+ ŌłØ 1 / (Zeff ) C+ ŌĆ”ŌĆ”ŌĆ”ŌĆ” (2)
rA- ŌłØ 1 / (Zeff)A ŌĆ”ŌĆ”ŌĆ”ŌĆ” (3) and
Where
Zeff is the effective nuclear charge and Zeff = ZŌłÆS
Dividing
the equation 2 by 3
rC+
/ rAŌłÆ = (Zeff AŌłÆ) / (Zeff C+) ŌĆ”ŌĆ”ŌĆ”ŌĆ” (4)
By
solving equation (1) & (4), rC+ and rA- can be
calculated
40. Explain the periodic trend of ionisation potential.
Periodic
variation in period:
ŌŚÅ
The ionisation energy usually increases along a period with few exceptions.When
we move from left to right along a period, the valence electrons are added to
the same shell, at the same time protons are added to the nucleus. This
successive increase of nuclear charge increases the electrostatic attractive
force on the valence electron and more energy is required to remove the valence
electron resulting in high ionisation energy.
Periodic
variation in group:
ŌŚÅ
The ionisation energy decreased down a group. As we move down a group, the
valence electron occupies new shells, the distance between the nucleus and the
valence electron increases.
ŌŚÅ
So, the nuclear forces of attraction on valence electron decreases and hence
ionisation energy also decreases down a group.
41. Explain the diagonal relationship.
On
moving diagonally across the periodic table, the second and third period
elements show certain similarities. Even though the similarity is not same as
we see in a group, it is quite pronounced in the following pair of elements.
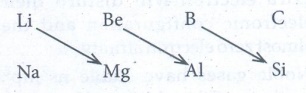
The
similarity in properties existing between the diagonally placed elements is
called 'diagonal relationship'.
42. Why the first ionisation enthalpy of sodium is lower that that of magnesium while its second ionisation enthalpy is higher than that of magnesium?
Size
of Mg is smaller than Na. Therefore the IE1 of Na is less than Mg.
Na loses an electron to become Na+, which acquires stable inert gas
configuration. From a stable electronic configuration removal of electron
requires more energy. Thus second ionisation enthalpy of Na is higher than that
of magnesium.
43. By using paulings method calculate the ionic radii of K+ and Cl- ions in the potassium chloride crystal. Given that dK+-Cl- = 3.14 Ū║
dk-cl- = rk+ + rcl-
= 3.14 Ū║ ŌĆ”ŌĆ”ŌĆ”ŌĆ”ŌĆ”..(1)
Electronic
configuration of K+ & Cl-
(1s2)(2s22s6)
(3s23p6)
Zeff = Z ŌłÆ S
Zeff
K+ = 19 ŌłÆ [(0.35├Ś7) + (0.85├Ś8) + (1.00├Ś2)]
= 19 ŌłÆ 11.25 = 7.75
Zeff
ClŌłÆ = 17 ŌłÆ 11.25 = 5.75
rk+ / rclŌłÆ
= (Zeff )ClŌłÆ
/ (Zeff)K+ = 5.75 / 7.75 = 0.74
rk+ = 0.74 rclŌłÆ ŌĆ”ŌĆ”ŌĆ”ŌĆ”.. (2)
Substituting
(2) in (1)
dk+ ŌłÆ dcl- = 0.74 rcl-
+ rcl- = 3.14 Ū║
1.74 rcl-= 3.14 Ū║
rcl-= 3.14 / 1.74 = 1.81 Ū║
rk+ = 0.74 rcl- =
0.74 ├Ś 1.81
rk+ = 1.33 Ū║
rk+ = 1.33 Ū║
rcl- = 1.81 Ū║
44. Explain the following, give appropriate reasons.
i. Ionisation potential of N is greater than that of O
ii. First ionisation potential of C-atom is greater than that of B atom, where as the reverse is true is for second ionisation potential.
iii. The electron affinity values of Be, Mg and noble gases are zero and those of N (0.02 eV) and P (0.80 eV) are very low
iv. The formation of F- (g) from F(g) is exothermic while that of O2-(g) from O (g) is endothermic.
Reasons :
i) Nitrogen with 1s2,
2s2, 2p3 electronic configuration has higher ionisation
energy (1402 kJ molŌłÆ1) than oxygen (1314 kJ mol-l). Since
the half filled electronic configuration is more stable, it requires higher
energy to remove an electron from 2p orbital of nitrogen. Whereas the removal
one 2p electron from oxygen leads to a stable half filled configuration. This
makes comparatively easier to remove 2p electron from
oxygen
ii) C (Z=6) electronic
configuration : 1s2, 2s2, 2p2
B(Z=5) electronic configuration :
1s2, 2s2, 2p1
Though the nuclear charge of C is
more than B, by losing one electron B acquires completely filled stable
configuration. From a stable configuration removal of electron requires more
energy. Hence, the second IE of B > C
iii)
It is due to the fact that beryllium with completely filled 2s orbital, is more
stable. The electronic configuration of beryllium (Z=4) in its ground state is
1s2, 2s2 nitrogen with (Z = 7) has 1s2, 2s2,
2p3 - half filled electronic configuration - more stable, Magnesium
has 1s2, 2s2, 2p6 3s2 completely
filled stable configuration phosphorus has 1s2, 2s2, 2p6
3s2 2p3 - half filled stable configuration, It
requires higher energy to remove an electron from stable half filled and
completely filled electronic configuration
(iv)
F(g) + eŌłÆ ŌåÆ F(g)ŌłÆ + Energy
Fluorine
readily accepts an electron to aquire stable inert gas configuration. Hence it
is exothermic.
O(g)
+ eŌłÆ ŌåÆ O 2ŌłÆ
This
is endothermic. The electron is added to a negative ion which is very small and
has high electron density. The additive electron leads to electron - electron
repulsion.
45. What is screening effect?
ŌŚÅ
In addition to the electrostatic forces of attraction between the nucleus and
the electrons, there exists repulsive forces among the electrons.
ŌŚÅ
The repulsive force between the inner shell electrons and the valence electrons
leads to a decrease in the electrostatic attractive forces acting on the
valence electrons by the nucleus.
ŌŚÅ
Thus, the inner shell electrons act as a shield between the nucleus and the
valence electrons. This effect is called shielding effect.
46. Briefly give the basis for pauling's scale of electronegativity.
ŌŚÅ
Electro-negativity is not a measurable quantity. However, a number of scales
are available to calculate its value.
ŌŚÅ
One such method was developed by Pauling's. He assigned arbitrary value of
electro-negativities for hydrogen and fluorine as 2.1 and 4.0 respectively.
ŌŚÅ
Based on this the electro-negativity values for other elements can be
calculated using the following expression
(ŽćA
ŌłÆ ŽćB)
= 0.182 ŌłÜEAB-(EAA*EBB)1/2
Where
EAB, EAA and EBB are the bond dissociation
energies of AB, A2 and B2 molecules respectively.
46. State the trends in the variation of electronegativity in group and periods.
Variation
in periods:
ŌŚÅ
The electronegativity generally increases across a period from left to right.
The atomic radius decreases in a period, as the attraction between the valence
electron and the nucleus increases.
ŌŚÅ
Hence the tendency to attract shared pair of electrons increases. Therefore,
electronegativty decreases.
Variation
in group:
As
we down the group, the electronegativity decreases.
Reason:
1)
Atomic radius Increases
2)
The attraction between the nucleus and valence electrons decreases.
Related Topics