Chapter: 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Grouping of Elements based on Electronic Configurations
Grouping
of Elements based on Electronic Configurations
In the modern periodic table, the elements are organised
in 7 periods and 18 groups based on the modern periodic law. The placement of
element in the periodic table is closely related to its outer shell electronic
configuration. Let us analyse the change in the electronic configuration of
elements along the periods and down the groups.
Variation of Electronic Configuration along the periods
We have already learnt that each period starts with the
element having general outer electronic configuration ns1 and ends
with ns2, np6 where n is the period number. The first
period starts with the filling of valence electrons in 1s orbital, which can
accommodate only two electrons. Hence, the first period has two elements,
namely hydrogen and helium. The second period starts with the filling of
valence electrons in 2s orbital followed by three 2p orbitals with eight
elements from lithium to neon. The third period starts with filling of valence
electrons in the 3s orbital followed by 3p orbitals. The fourth period starts
with filling of valence electrons from 4s orbital followed by 3d and 4p
orbitals in accordance with Aufbau principle. Similarly, we can explain the
electronic configuration of elements in the subsequent periods (Table 3.10).
Table 3.10 Electronic configuration of elements in a period
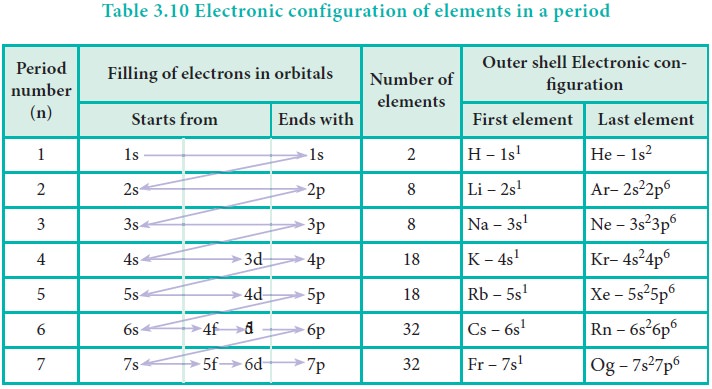
In the fourth period the filling of 3d orbitals starts
with scandium and ends with zinc. These 10 elements are called first transition
series. Similarly 4d, 5d and 6d orbitals are filled in successive periods and
the corresponding series of elements are called second, third and fourth
transition series respectively.
In the sixth period the filling of valence electrons
starts with 6s orbital followed by 4f, 5d and 6p orbitals. The filling up of 4f
orbitals begins with Cerium (Z=58) and ends at Lutetium (Z=71). These 14
elements constitute the first inner-transition series called Lanthanides.
Similarly, in the seventh period 5f orbitals are filled, and it's -14 elements
constitute the second inner-transition series called Actinides. These two
series are placed separately at the bottom of the modern periodic table.
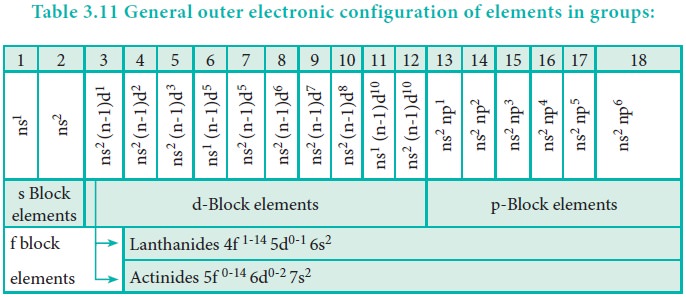
Variation of Electronic Configuration in the Groups:
Elements of a group have similar electronic configuration
in the outer shell. The general outer electronic configurations for the 18
groups are listed in the Table 3.11. The groups can be combined as s, p, d and
f block elements on the basis of the orbital in which the last valence electron
enters.
The elements of group 1 and group 2 are called s-block
elements, since the last valence electron enters the ns orbital. The group 1
elements are called alkali metals while the group 2 elements are called alkaline
earth metals. These are soft metals and possess low melting and boiling points
with low ionisation enthalpies. They are highly reactive and form ionic
compounds. They are highly electropositive in nature and most of the elements
imparts colour to the flame. We will study the properties of these group
elements in detail in subsequent chapters.
The elements of groups 13 to 18 are called p-block
elements or representative elements and have a general electronic configuration
ns2, np1-6. The elements of the group 16 and 17 are
called chalcogens and halogens respectively. The elements of 18th
group contain completely filled valence shell electronic configuration (ns2,
np6) and are called inert gases or nobles gases. The elements of
p-block have high negative electron gain enthalpies. The ionisation energies
are higher than that of s-block elements. They form mostly covalent compounds
and shows more than one oxidation states in their compounds.
The elements of the groups 3 to 12 are called d-block
elements or transition elements with general valence shell electronic
configuration ns1-2, (n-1)d1-10. These elements also show
more than one oxidation state and form ionic, covalent and co-ordination
compounds. They can form interstitial compounds and alloys which can also act
as catalysts. These elements have high melting points and are good conductors
of heat and electricity.
The lanthanides (4f1-14, 5d0-1, 6s2)
and the actinides (5f0-14, 6d0-2, 7s2) are
called f-block elements. These elements are metallic in nature and have high
melting points. Their compounds are mostly coloured. These elements also show
variable oxidation states.
Table 3.11 General outer electronic configuration of elements in groups:
Related Topics