Chapter: 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Ionisation enthalpy or Ionisation Energy
Ionisation energy
It is defined as the minimum amount of energy required to remove the most loosely bound electron from the valence shell of the isolated neutral gaseous atom in its ground state. It is expressed in kJ mol-1 or in electron volts (eV).
M(g) + IE1 → M+(g) + 1 e-
Where IE1 represents the first ionisation energy.
Successive Ionisation energies
The minimum amount of energy required to remove an electron from a unipositive cation is called second ionisation energy. It is represented by the following equation.
M+(g) + IE2 → M2+(g)+ 1 e-
In this way we can define the successive ionisation energies such as third, fourth etc.
The total number of electrons are less in the cation than the neutral atom while the nuclear charge remains the same. Therefore the effective nuclear charge of the cation is higher than the corresponding neutral atom. Thus the successive ionisation energies, always increase in the following order
IE1 < IE2 < IE3 < .....
Periodic Trends in Ionisation Energy
The ionisation energy usually increases along a period with few exceptions. As discussed earlier, when we move from left to right along a period, the valence electrons are added to the same shell, at the same time protons are added to the nucleus. This successive increase of nuclear charge increases the electrostatic attractive force on the valence electron and more energy is required to remove the valence electron resulting in high ionisation energy.
Let us consider the variation in ionisation energy of second period elements. The plot of atomic number vs ionisation energy is given below.
In the following graph, there are two deviation in the trends of ionisiation energy. It is expected that boron has higher ionisation energy than beryllium since it has higher nuclear charge. However, the actual ionisation energies of beryllium and boron are 899 and 800 kJ mol-1 respectively contrary to the expectation. It is due to the fact that beryllium with completely filled 2s orbital, is more stable than partially filled valence shell electronic configuration of boron. (2s2,2p1)
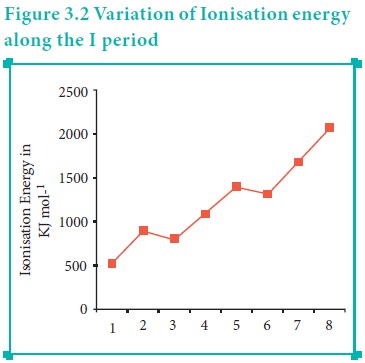
The electronic configuration of beryllium (Z=4) in its ground state is 1s2, 2s2 and that of boran (Z = 5) 1s2 2s2 2p1
Similarly, nitrogen with 1s2, 2s2, 2p3 electronic configuration has higher ionisation energy (1402 kJ mol-1) than oxygen (1314 kJ mol-1). Since the half filled electronic configuration is more stable, it requires higher energy to remove an electron from 2p orbital of nitrogen. Whereas the removal one 2p electron from oxygen leads to a stable half filled configuration. This makes comparatively easier to remove 2p electron from oxygen.
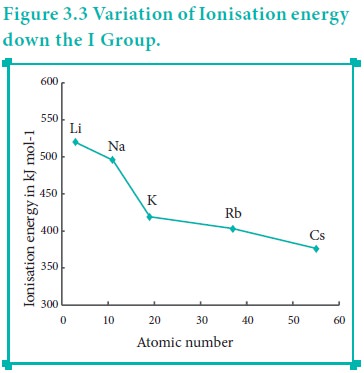
Periodic variation in group
The ionisation energy decreases down a group. As we move down a group, the valence electron occupies new shells, the distance between the nucleus and the valence electron increases. So, the nuclear forces of attraction on valence electron decreases and hence ionisation energy also decreases down a group.
Ionisation energy and shielding effect
As we move down a group, the number of inner shell electron increases which in turn increases the repulsive force exerted by them on the valence electrons, i.e. the increased shielding effect caused by the inner electrons decreases the attractive force acting on the valence electron by the nucleus. Therefore the ionisation energy decreases.
Let us understand this trend by considering the ionisation energy of alkali metals.
Related Topics