Chapter: 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Nomenclature of Elements with Atomic Number Greater than 100
Nomenclature
of Elements with Atomic Number Greater than 100
Usually, when a new element is discovered, the discoverer
suggests a name following IUPAC guidelines which will be approved after a
public opinion. In the meantime, the new element will be called by a temporary
name coined using the following IUPAC rules, until the IUPAC recognises the new
name.
1. The name was derived directly from the atomic number of
the new element using the following numerical roots.
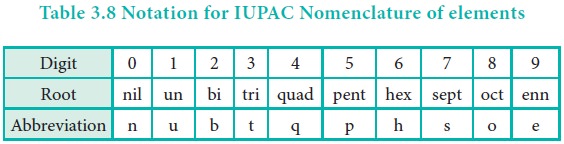
2. The numerical roots corresponding to the atomic number
are put together and ŌĆśiumŌĆÖ is added as suffix
3. The final ŌĆśnŌĆÖ of ŌĆśennŌĆÖ is omitted when it is written
before ŌĆśnilŌĆÖ (enn + nil = enil) similarly the final ŌĆśi' of ŌĆśbiŌĆÖ and ŌĆśtriŌĆÖ is
omitted when it written before ŌĆśiumŌĆÖ (bi + ium = bium; tri + ium = trium)
4. The symbol of the new element is derived from the first
letter of the numerical roots. The following table illustrates these facts.
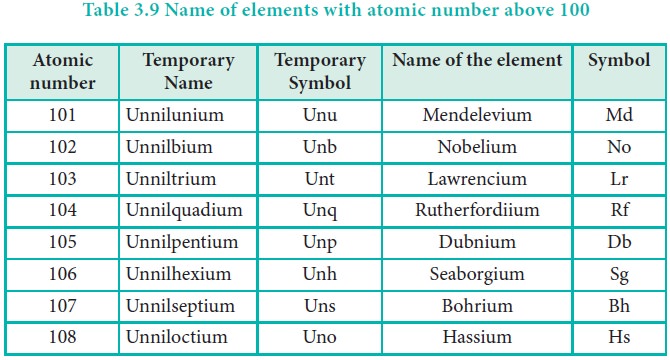
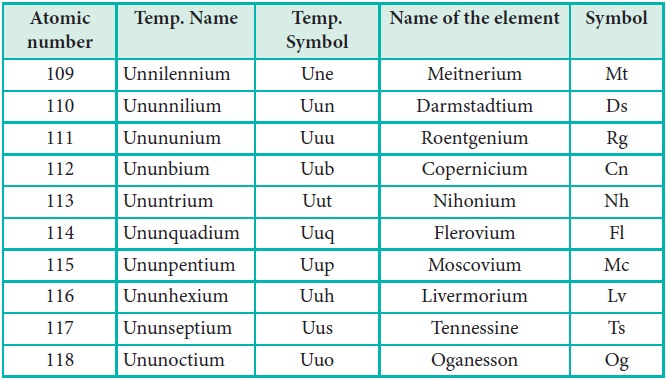
Related Topics