Modern Periodic Table | Chemistry - Anomalous properties of second period elements | 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Chapter: 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Anomalous properties of second period elements
Anomalous properties of second period elements:
As we know, the elements of the same group show similar physical and chemical properties. However, the first element of each group differs from other members of the group in certain properties. For example, lithium and beryllium form more covalent compounds, unlike the alkali and alkali earth metals which predominantly form ionic compounds. The elements of the second period have only four orbitals (2s & 2p) in the valence shell and have a maximum co-valence of 4, whereas the other members of the subsequent periods have more orbitals in their valence shell and shows higher valences. For example, boron forms BF4ŌĆōand aluminium forms AlF63ŌĆō
Diagonal Relationship
On moving diagonally across the periodic table, the second and third period elements show certain similarities. Even though the similarity is not same as we see in a group, it is quite pronounced in the following pair of elements.
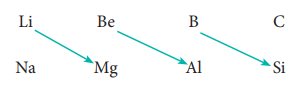
The similarity in properties existing between the diagonally placed elements is called ŌĆśdiagonal relationshipŌĆÖ.
Related Topics