Chapter: 11th Chemistry : UNIT 3 : Periodic Classification of Elements
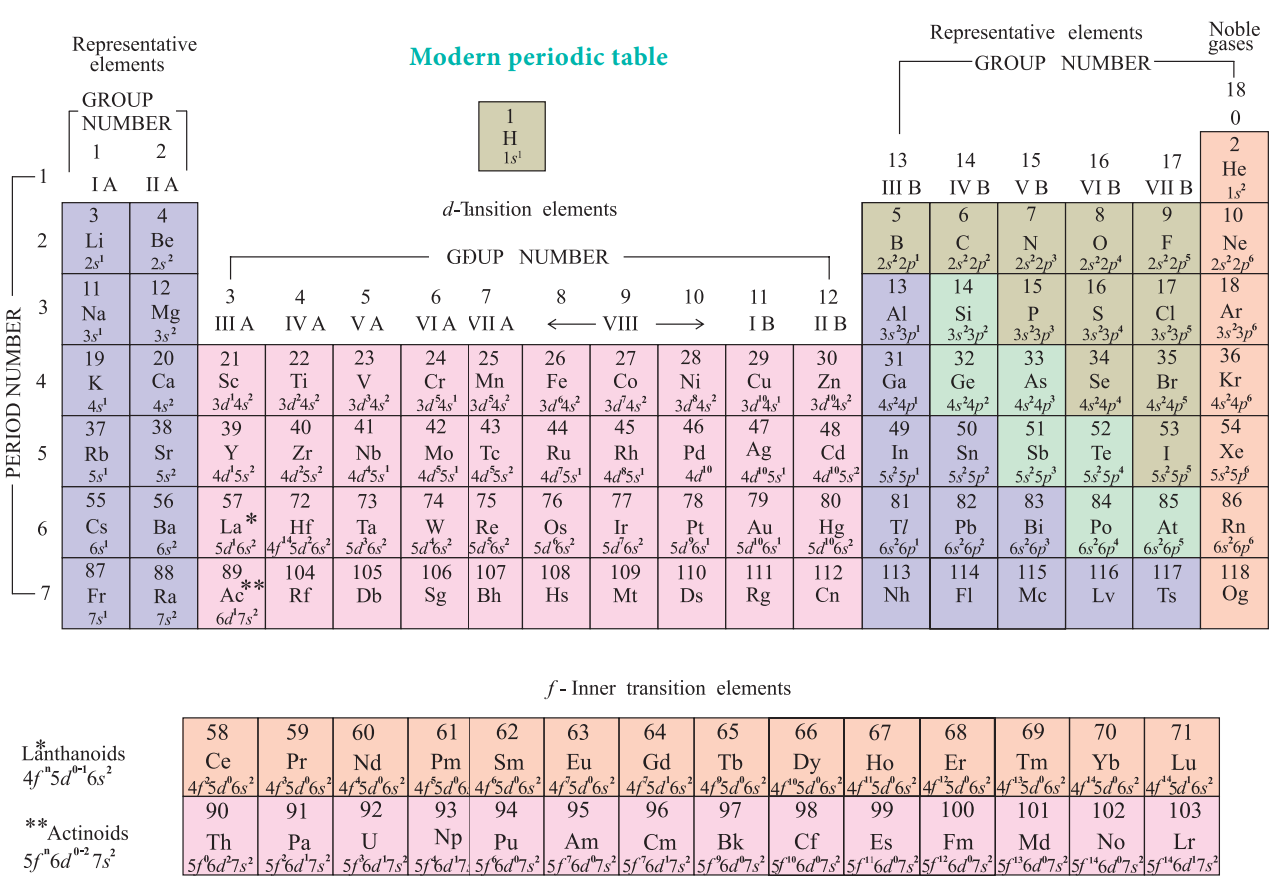
Modern Periodic Table
Modern
Periodic Table
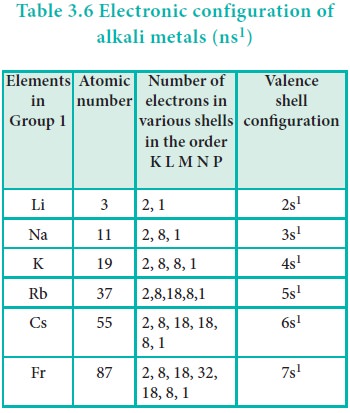
The physical and chemical properties of the elements are
correlated to the arrangement of electrons in their outermost shell (valence
shell). Different elements having similar outer shell electronic configuration
possess similar properties. For example, elements having one electron in their
valence shell s-orbital possess similar physical and chemical properties. These
elements are grouped together in the modern periodic table as first group
elements.
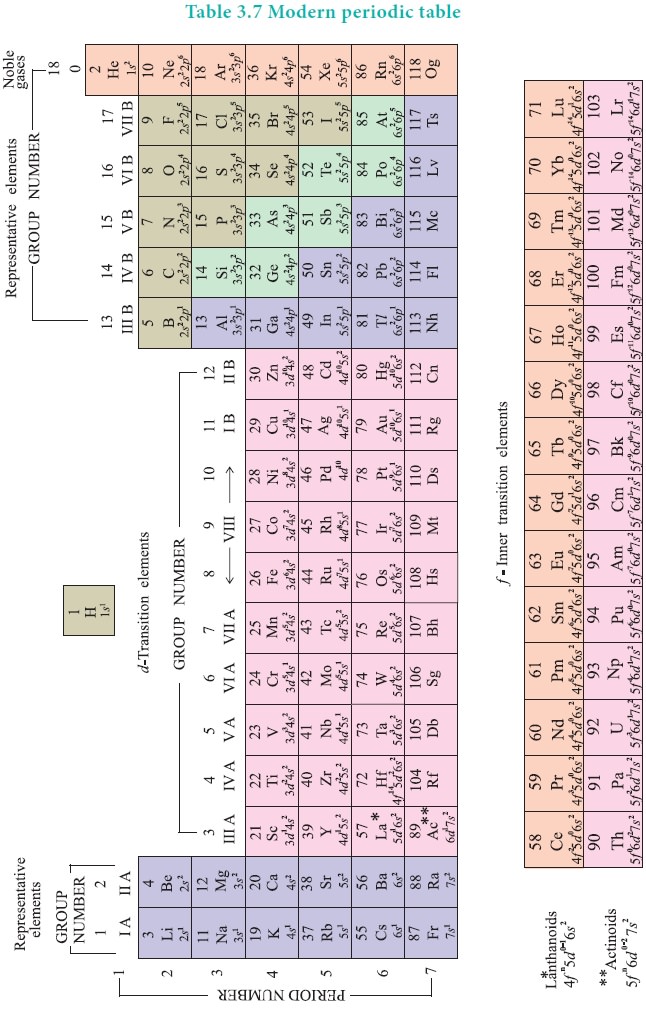
Similarly, all the elements are arranged in the modern
periodic table which contains 18 vertical columns and 7 horizontal rows. The
vertical columns are called groups and the horizontal rows are called periods.
Groups are numbered 1 to 18 in accordance with the IUPAC recommendation which
replaces the old numbering scheme IA to VIIA, IB to VIIB and VIII.
Each period starts with the element having general outer
electronic configuration ns1 and ends with np6. Here ‘n’
corresponds to the period number (principal quantum number). The aufbau
principle and the electronic configuration of atoms provide a theoretical foundation
for the modern periodic table.
Related Topics