Chemistry - Classification of Elements | 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Chapter: 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Classification of Elements
Classification
of Elements
During the 19th century, scientists have
isolated several elements and the list of known elements increased. Currently,
we have 118 known elements. Out of 118 elements, 92 elements with atomic
numbers 1 to 92 are found in nature. Scientists have found out there are some
similarities in properties among certain elements. This observation has led to
the idea of classification of elements based on their properties. In fact,
classification will be beneficial for the effective utilization of these
elements. Several attempts were made to classify the elements. However,
classification based on the atomic weights led to the construction of a proper
form of periodic table.
In 1817, J. W. D├Čbereiner classified some elements such as
chlorine, bromine and iodine with similar chemical properties into the group of
three elements called as triads. In triads, the atomic weight of the middle
element nearly equal to the arithmetic mean of the atomic weights of the
remaining two elements. However, only a limited number of elements can be
grouped as triads.
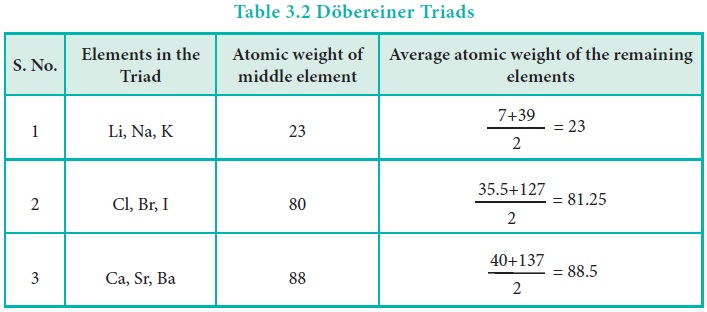
This concept can not be extended to some triads which have
nearly same atomic masses such as [Fe, Co, Ni], [Ru, Rh, Pd] and [Os, Ir, Pt].
In 1862, A. E. B. de Chancourtois reported a correlation
between the properties of the elements and their atomic weights. He said ŌĆśthe
properties of bodies are the properties of numbersŌĆÖ. He intended the term
numbers to mean the value of atomic weights. He designed a helix by tracing at
an angle 45˚ to the vertical axis of a cylinder with circumference of 16 units.
He arranged the elements in the increasing atomic weights along the helix on
the surface of this cylinder. One complete turn of a helix corresponds to an
atomic weight increase of 16. Elements which lie on the 16 equidistant vertical
lines drawn on the surface of cylinder shows similar properties. This was the
first reasonable attempt towards the creation of periodic table. However, it
did not attract much attention.
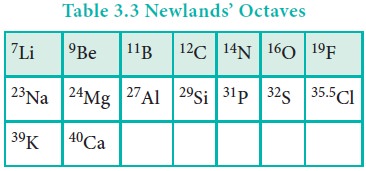
In 1864, J. Newland made an attempt to classify the
elements and proposed the law of octaves. On arranging the elements in the
increasing order of atomic weights, he observed that the properties of every
eighth element are similar to the properties of the first element. This law
holds good for lighter elements up to calcium.
Mendeleev's Classification
In 1868, Lothar Meyer had developed a table of the
elements that closely resembles the modern periodic table. He plotted the
physical properties such as atomic volume, melting point and boiling point
against atomic weight and observed a periodical pattern.
During same period Dmitri Mendeleev independently proposed
that ŌĆ£the properties of the elements are the periodic functions of their atomic
weightsŌĆØ and this is called periodic law. Mendeleev listed the 70 known
elements at that time in several vertical columns in order of increasing atomic
weights. Thus, Mendeleev constructed the first periodic table based on the
periodic law.
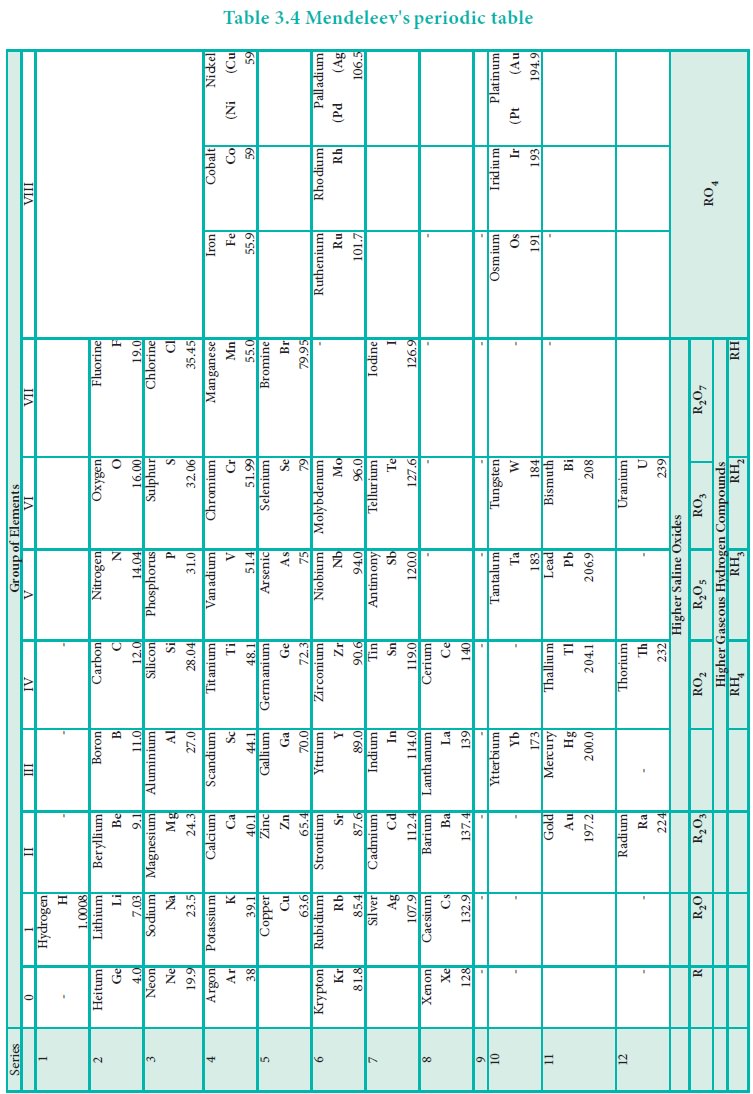
As shown in the periodic table, he left some blank spaces
since there were no known elements with the appropriate properties at that
time. He and others predicted the physical and chemical properties of the
missing elements. Eventually these missing elements were discovered and found
to have the predicted properties. For example, Gallium (Ga) of group and
germanium (Ge) of group IV were unknown at that time. But Mendeleev predicted
their existence and properties. He referred the predicted elements as
eka-aluminium and eka-silicon. After discovery of the actual elements, their
properties were found to match closely to those predicted by Mendeleev (Table
3.4 ).
Anomalies of MendeleevŌĆÖs Periodic Table
Some elements with similar properties were placed in
different groups and those with dissimilar properties were placed in same
group.
Example: Tellurium (127.6) was placed in VI group but
Iodine (127.0) was placed in VII group.
Similarly elements with higher atomic weights were placed
before lower atomic weights based on their properties in contradiction to his
periodic law. Example 59Co27 was placed before 58.7Ni28
Related Topics