Mathematics - Applications of First Order Ordinary Differential Equations | 12th Maths : UNIT 10 : Ordinary Differential Equations
Chapter: 12th Maths : UNIT 10 : Ordinary Differential Equations
Applications of First Order Ordinary Differential Equations
Applications of
First Order Ordinary Differential Equations
The
subject of differential equations has vast applications in solving real world
problems. The solutions of the differential equations are used to predict the
behaviors of the system at a future time, or at an unknown location. In several
problems, the rate at which a quantity changes is a given function of the
quantity and /or the time. The objective is to find the quantity itself. If x denotes the amount of the quantity
present at time t , then the
instantaneous rate at which the quantity changes at time t is dx/dt . This leads
to a differential equation of the form dx/dt
=
f ( x, t) . In this section
we shall consider this type of problems only. Further, by rate, we mean the
instantaneous rate only.
1. Population growth
Now, we
consider the growth of a population (for example, human, an animal, or a
bacteria colony) as a function of time t
.
Let x(t)
be the size of the population at any time t
. Although x(t) is integer-valued, we approximate x(t) as a differentiable
function and techniques of differential equation can be applied to determine x(t)
. Assume that population grows at a rate directly proportional to the amount of
population present at that time. Then, we obtain
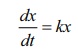
dx/dt = kx ,
where k is the constant of proportionality . … (1)
Here k > 0 , since the population always
increases.
The
solution of the differential equation is x(t) = Cekt
, where C is a constant of
integration. The values of C and k are determined with the help of
initial conditions. Thus, the population increases exponentially with time.
This law of population growth is called Malthusian law.
Example 10.27
The
growth of a population is proportional to the number present. If the population
of a colony doubles in 50 years, in how many years will the population become
triple?
Solution
Let x(t)
be the population at time t . Then dx/dt = kx
.
By separating the variables, we obtain dx/x = kdt .
Integrating
on both sides, we get, log |x| =
kt + log |C| or x = Cekt , where C is an arbitrary constant.
Let x0 be the population when t = 0 and obtain C = x0 .
Thus, we
get x = x0ekt .
Now x = 2x0 , when t =
50 and thus, k =
1/50 log 2 .
Hence, x = x0
2t/50 is the population at any time t.
Assume
that the population is tripled in t1
years.
That is,
x = 3x0 , when t =
t1 .
Thus, t1
= 50 (log3/ log2).Therefore, the population is tripled in 50(log3/ log2) years.
2. Radioactive decay
The
nucleus of an atom consists of combinations of protons and neutrons. Many of
these combinations of protons and neutrons are unstable, that is the atoms
decay or transmute into the atoms of another substance. Such nuclei are said to
be radioactive.
It is
assumed that the rate dA/dt at which the nuclei of a substance decays
is proportional to the amount A(t) of the substance remaining at time t.
Thus,
the required differential equation is
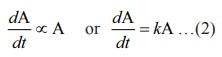
dA/dt ∝ A or dA/dt = kA …(2),
where k
is the constant of proportionality. Here k
<
0 , since decay occurs.
Remarks
From
equations (1) and (2), we see that the differential equations are the same, but
the difference is only in the interpretations of the symbols and the constants
of proportionality. For growth as we expect in (1), k > 0 and in the case of (2) for decay,
k < 0 .
A single differential
equation can serve as a mathematical model for many different phenomena.
Example 10.28
A
radioactive isotope has an initial mass 200mg , which two years later is 50mg .
Find the expression for the amount of the isotope remaining at any time. What
is its half-life? (half-life means the time taken for the radioactivity of a
specified isotope to fall to half its original value).
Solution
Let A be the mass of the isotope remaining
after t years, and let −k be the constant of proportionality,
where k >
0 . Then the rate of decomposition is modeled by dA/dt = −kA , where the minus sign indicates that the mass is
decreasing. It is a separable equation. Separating the variables, we get dA / A
= −kdt
Integrating
on both sides, we get log |A| = −kt + log
|C| or A =
Ce−kt .
Given
that the initial mass is 200mg. That is, A = 200 when t = 0 and thus, C =
200 .
Thus, we
get A =
200e−kt .
Also, A =
150 when t =
2 and therefore, k = 1/2
log (4/3).
Hence, 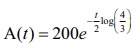 is the
mass of isotope remaining after t
years.
is the
mass of isotope remaining after t
years.
The
half-life th is the time
corresponding to A = 100mg .
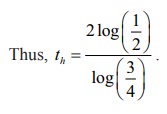
3. Newton’s Law of cooling/warming
Consider
pouring a 150° cup of coffee and kept it on the table in an 80°C room.

What
happens to the temperature of the coffee? We observe that the cup of coffee
will cool off until it reaches the room temperature.
Now consider
taking a 35° glass of cold water from the refrigerator and kept it on
the table in an 80°C room. What happens to the
temperature of the cold water? Similarly, we can observe the water will warm up
until it reaches room temperature.

According
to Newton’s
law of cooling or warming, the rate at which the temperature of a
body changes is proportional to the difference between the temperature of the
body and the temperature of the surrounding medium the so-called ambient
temperature. If T (t) represents
the temperature of a body at time t , Tm the temperature of the surrounding medium, and dT/dt the rate at which the temperature
of the body changes, then Newton’s law of cooling(or warming) is dT/dt ∝ T
−Tm or dT/dt = k
(
T − Tm
)
, where k is constant of proportionality.
In either case, cooling or warming, if Tm
is constant, it stands to reason that k
<
0 .
Example 10.29
In a
murder investigation, a corpse was found by a detective at exactly 8 p.m. Being
alert, the detective also measured the body temperature and found it to be 70oF.
Two hours later, the detective measured the body temperature again and found it
to be 60oF. If the room temperature is 50oF, and assuming
that the body temperature of the person before death was 98.6oF, at
what time did the murder occur?
[log ( 2.43
) = 0.88789; log ( 0.5 ) = −0.69315 ]
Solution
Let T be the temperature of the body at any
time t and with time 0 taken to be 8
p.m.
By
Newton’s law of cooling,
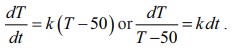
Integrating
on both sides, we get log |50 – T| = kt
+ log C or 50 − T = Cekt .
When t =
0, T = 70 , and so C = −20
When t =
2,T = 60 , we have −10 = −20ek2.
Thus, k = 1/2 log(1/2) .
Hence,
the solution is 50 − T =
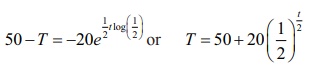
Now, we
would like to find the value of t, for which T (t) = 98.6 , and
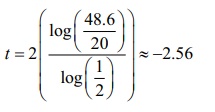
It
appears that the person was murdered at about 5.30 p.m.
4. Mixture problems
Mixing
problems occur quite frequently in chemical industry. Now we explain how to
solve the basic model involving a single tank.
A
substance S is allowed to flow into a
certain mixture in a container at a constant rate, and the mixture is kept
uniform by stirring. Further, in one such situation, this uniform mixture
simultaneously flows out of the container at another rate. Now we seek to
determine the quantity of the substance S
present in the mixture at time t
.
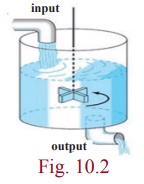
Letting x to denote the amount of S present at time t and the derivative dx/dt
to denote the rate of change of x
with respect to t . If IN denotes the rate at which S enters the mixture and OUT denotes the rate at which it leaves,
then we have the equation dx/dt =
IN – OUT
Example 10.30
A tank
contains 1000 litres of water in which 100 grams of salt is dissolved. Brine (Brine is a high-concentration solution of salt (usually sodium chloride) in water)
runs in a rate of 10 litres per minute,
and each litre contains 5grams of dissolved salt. The mixture of the tank is
kept uniform by stirring. Brine runs out at 10 litres per minute. Find the
amount of salt at any time t .
Solution
Let x(t)
denote the amount of salt in the tank at time t . Its rate of change is dx/dt = in
flow rate - out flow rate
Now,
5 grams times 10 litres gives an inflow of 50 grams of salt. Also, the out flow
of brine is 10 litres per minute. This is 10 / 1000 =
0.01 of the total brine content in the tank. Hence, the outflow of salt is 0.01
times x(t) , that is 0.01x(t) .
Thus the
differential equation for the model is dx/dt
=
50 −
0.01x = −0.01(
x − 5000)
This can
be written as
dx / x–5000 =
−(0.01)dt
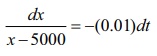
Integrating
both sides, we obtain log | x –
5000 | = −0.01t + log C
or x − 5000 = Ce−0.01t or x =
5000 +
Ce−0.01t
Initially,
when t = 0, x = 100 , so 100 = 5000 + C
.Thus, C = −4900
.
Hence,
the amount of the salt in the tank at time t
is x = 5000 − 4900e−0.01t .
Related Topics