Chapter: Modern Analytical Chemistry: Gravimetric Methods of Analysis
Theory and Practice of Precipitation Gravimetry: Filtering the Precipitate
Filtering the Precipitate
After precipitation and digestion are complete, the precip-
itate is separated from solution
by filtration using
either filter paper
or a filtering cru-
cible. The most common filtering
medium is cellulose-based filter paper, which is
classified according to its filtering speed, its size,
and its ash content on ignition. Fil- tering speed is a function of the paper’s
pore size, which
determines the particle sizes retained by the
filter. Filter paper
is rated as fast (retains particles > 20–25
μm), medium fast (retains
particles > 16 μm), medium
(retains particles >
8 μm), and slow
(retains particles > 2–3 μm). The proper
choice of filtering speed is important. If the filtering speed is too fast, the precipitate may pass through
the filter paper re-
sulting in a loss of precipitate. On the other
hand, the filter
paper can become
clogged when using
a filter paper
that is too slow.
Filter paper is hygroscopic and is not easily dried
to a constant weight. As a re- sult, in a quantitative procedure the filter paper must be removed
before weighing the precipitate. This is accomplished by carefully igniting
the filter paper.
Following ignition, a residue
of noncombustible inorganic ash remains that
contributes a posi- tive determinate error to the precipitate’s final mass. For quantitative analytical pro- cedures a low-ash
filter paper must be used.
This grade of filter paper
is pretreated by washing
with a mixture of HCl and HF to remove inorganic materials. Filter paper classed as quantitative has
an ash content
of less than
0.010% w/w. Qualita- tive filter paper typically has a maximum
ash content of 0.06% w/w.
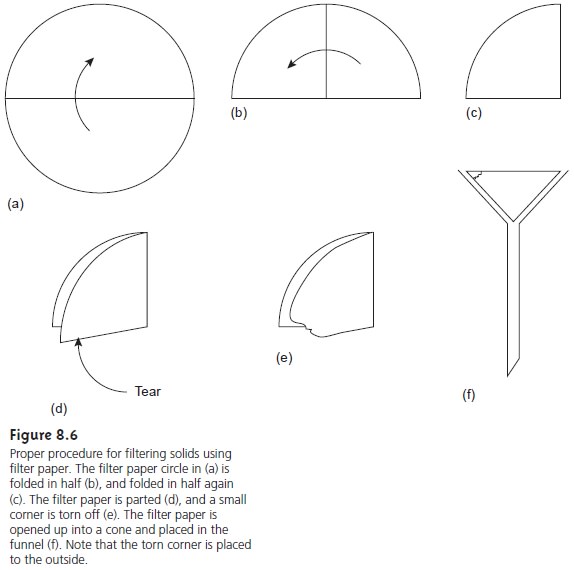
Filtering is accomplished by folding the
filter paper into
a cone, which
is then placed in a long-stem funnel (Figure 8.6).
A seal between the filter
cone and the funnel is formed by dampening the paper with water and pressing the paper to the
wall of the funnel. When
properly prepared, the
stem of the
funnel will fill
with the solution being
filtered, increasing the
rate of filtration. Filtration is accomplished by the force of gravity.
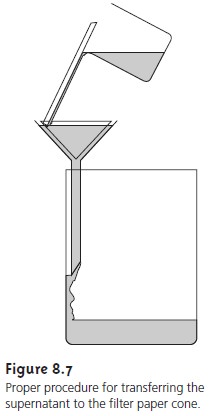
The precipitate is transferred to the filter in several
steps (Figure 8.7). The first step is to decant
the majority of the supernatant through the filter paper
without transferring the precipitate. This is done to prevent
the filter paper
from becoming clogged at the beginning of the filtration process. Initial rinsing
of the precipitate is done in the beaker
in which the precipitation was performed. These
rinsings are also decanted through the filter
paper. Finally, the
precipitate is transferred onto the filter paper
using a stream
of rinse solution. Any precipitate clinging
to the walls of the beaker
is transferred using
a rubber policeman (which is simply
a flexible rubber spatula attached to the end of a glass
stirring rod).
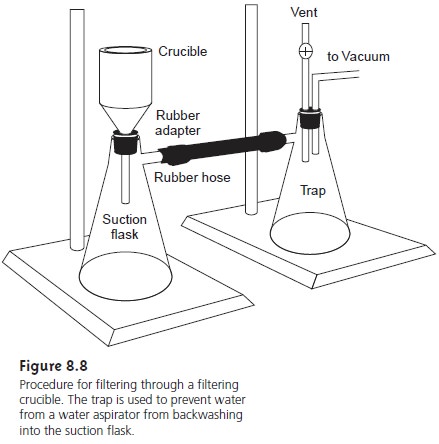
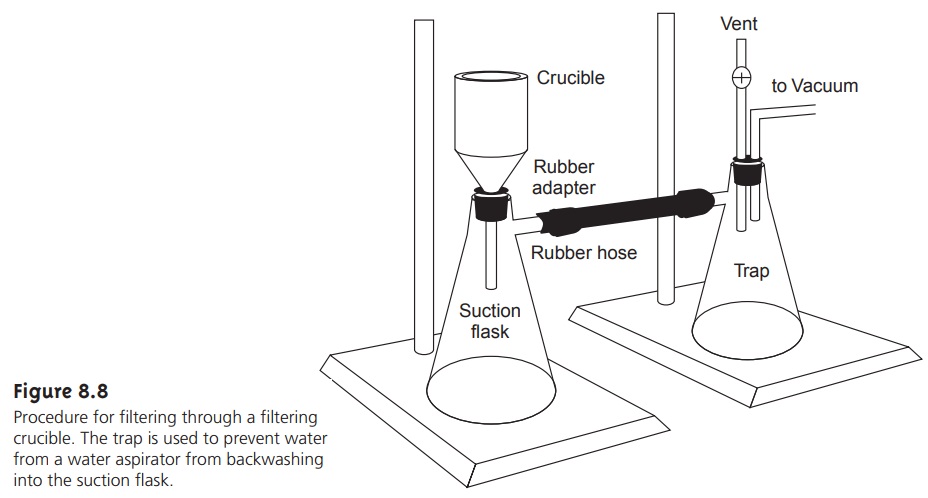
An alternative method
for filtering the
precipitate is a filtering crucible (Fig- ure 8.8). The most common
is a fritted glass crucible
containing a porous
glass disk filter. Fritted
glass crucibles are classified by their porosity:
coarse (retaining particles > 40–60 μm), medium (retaining particles > 10–15 μm), and fine (re- taining particles > 4–5.5
μm). Another type
of filtering crucible is the Gooch
cru- cible, a porcelain crucible with a perforated bottom.
A glass fiber
mat is placed in the crucible
to retain the precipitate, which
is transferred to the crucible
in the same manner
described for filter
paper. The supernatant is drawn through
the crucible with
the assistance of suction from
a vacuum aspirator or pump.
Related Topics