Chapter: Biochemistry: Lipid Metabolism
Synthesis of Acylglycerols and Compound Lipids
Synthesis of Acylglycerols and
Compound Lipids
Other lipids, including triacylglycerols, phosphoacylglycerols, and steroids, are derived from fatty acids and metabolites of fatty acids, such as acetoacetyl-CoA. Free fatty acids do not occur in the cell to any great extent; they are normally found incorporated in triacylglycerols and phosphoacylglycerols.
The biosynthesis of these two types of compounds takes
place principally on the ER of liver cells or fat cells (adipocytes).
Triacylglycerols
The glycerol portion of lipids is derived from glycerol-3-phosphate, a compound available from glycolysis. In liver and kidney, another source is glycerol released by degradation of acylglycerols. An acyl group of a fatty acid is transferred from an acyl-CoA. The products of this reaction are CoA-SH and a lysophosphatidate (a monoacylglycerol phosphate) (Figure 21.19). The acyl group is shown as esterified at carbon atom 2 (C-2) in this series of equations, but it is equally likely that it is esterified at C-1.
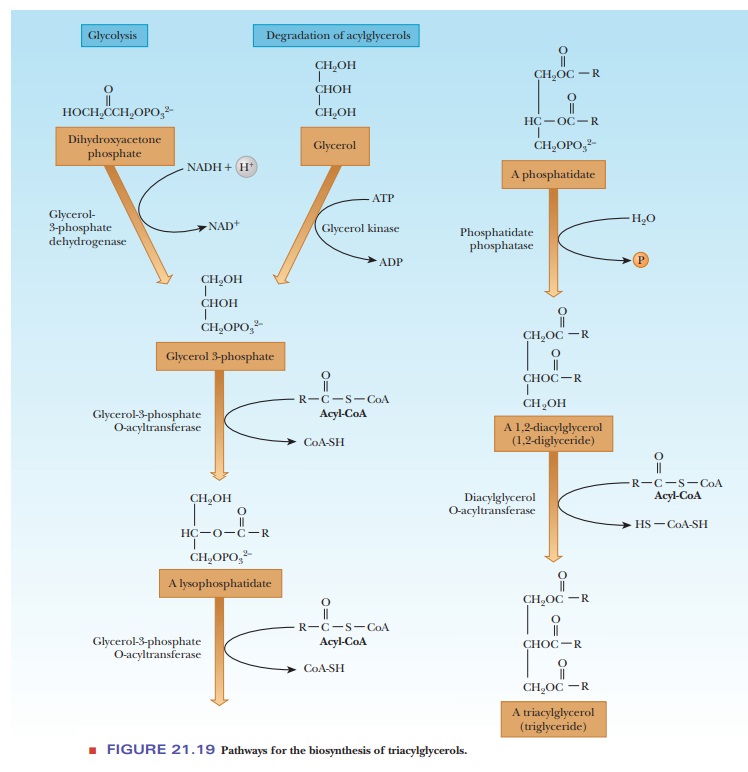
A second acylation reaction
takes place, catalyzed by the same enzyme, producing a phosphatidate (a diacylglyceryl phosphate). Phosphatidates occur in
membranes and are precursors of other phospholipids. The phosphate group of the
phosphatidate is removed by hydrolysis, producing a diacylglycerol. A third acyl group is added in a reaction in which
the source of the acyl group is an acyl-CoA rather than the free fatty acid.
How does the biosynthesis of phosphoacylglycerols take place?
Phosphoacylglycerols (phosphoglycerides) are based on
phosphatidates, with the phosphate group esterified to another alcohol,
frequently a nitrogen-containing alcohol such as ethanolamine. The conversion
of phosphatidates to other phospholipids frequently requires the presence of
nucleoside triphosphates, particularly cytidine
triphosphate(CTP). The role of CTP depends on the type of organism, because
the details of the biosynthetic pathway are not the same in mammals and
bacteria. We shall use a comparison of the synthesis of
phosphatidylethanolamine in mammals and in bacteria (Figure 21.20) as a case
study of the kinds of reactions commonly encountered in phosphoglyceride
biosynthesis.
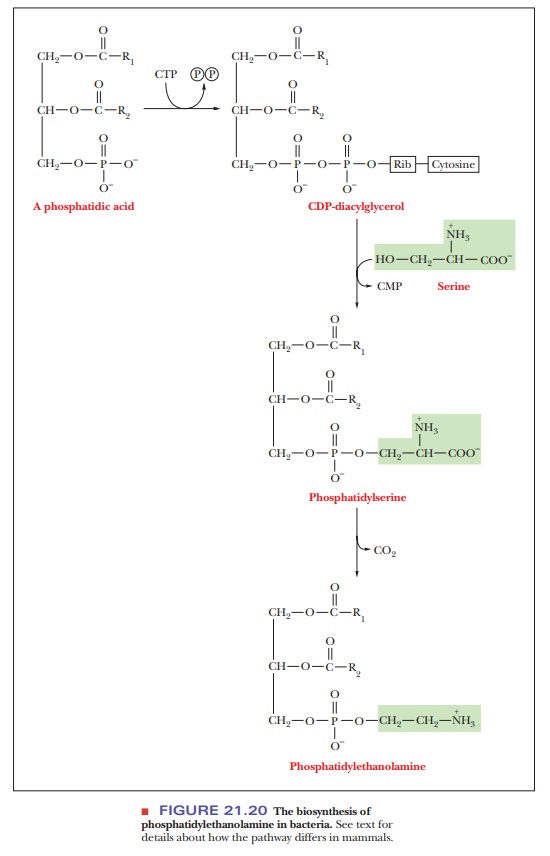
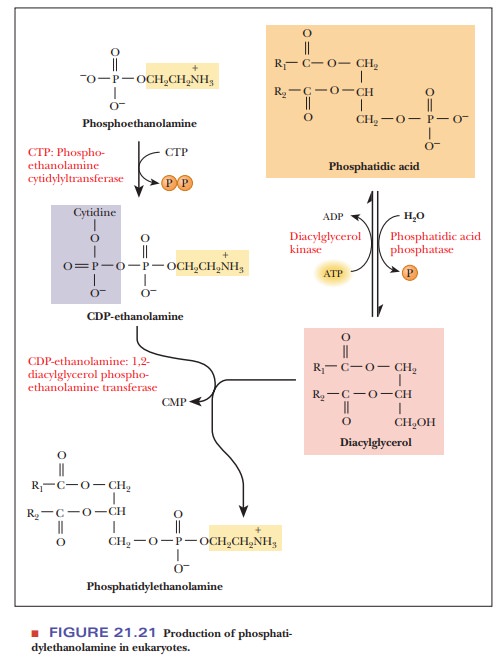
In bacteria, CTP reacts with phosphatidate to produce cytidine diphospho-diacylglycerol (a CDP diglyceride). The CDP diglyceride reacts with serine to form phosphatidylserine. Phosphatidylserine is then decarboxylated to give phosphatidylethanolamine. In eukaryotes, the synthesis of phosphatidylethanol-amine requires two preceding steps in which the component parts are pro-cessed (Figure 21.21). The first of these two steps is the removal by hydrolysis of the phosphate group of the phosphatidate, producing a diacylglycerol; the second step is the reaction of ethanolamine phosphate with CTP to produce pyrophosphate (PPi) and cytidine diphosphate ethanolamine (CDP-ethanolamine). The CDP-ethanolamine and diacylglycerol react to form phosphatidylethanolamine.
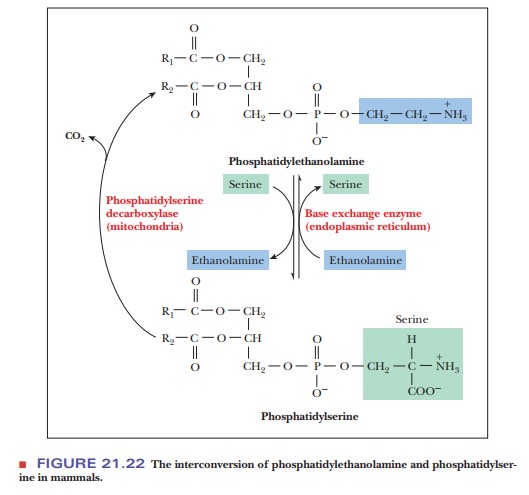
In mammals, phosphatidylethanolamine can be produced another way.
Alcohol exchange from serine to ethanolamine allows the interconversion of
phosphatidylethanolamine with phosphatidylserine (Figure 21.22).
How does the biosynthesis of sphingolipids take place?
The structural basis of sphingolipids is not glycerol but sphingosine, a long-chain amine. The precursors of sphingosine are palmitoyl-CoA and the amino acid serine, which react to produce dihydrosphingosine.
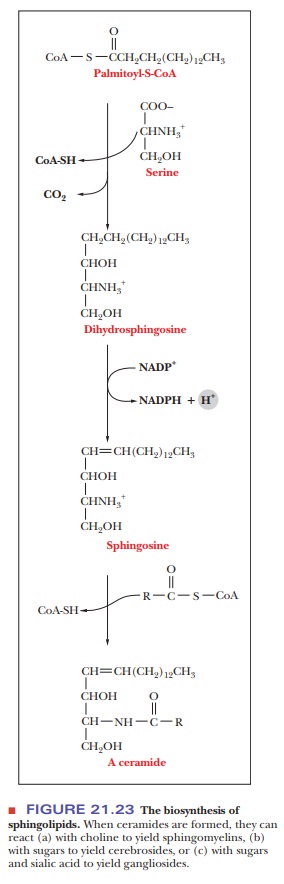
The carboxyl group of the serine is lost as CO2
in the course of this reaction (Figure 21.23). An oxidation reaction introduces
a double bond, with sphingosine as the resulting compound. Reaction of the
amino group of sphingosine with another acyl-CoA to form an amide bond results
in an N-acylsphingosine, also called
a ceramide. Ceramides in turn are
the parent compounds of sphingomyelins, cerebrosides, and gangliosides.
Attachment of phosphorylcholine to the primary alcohol group of a ceramide
produces a sphingomyelin, whereas
attachment of sugars such as glucose at the same site produces cerebrosides. Gangliosides are formed
from ceramides by attachment of oligosaccharides that contain a sialic acid
residue, also at the primary alcohol group.
Summary
Most compound lipids such as triacylglycerols,
phosphoacylglycerols, and sphingolipids, have fatty acids as precursors.
Fatty acids are linked to a backbone molecule, such as glycerol for triacyl-glycerols or phosphoacylglycerols, or sphingosine for sphingolipids. Other moieties are added to give rise to specific compounds.
Related Topics