Chapter: Introduction to Human Nutrition: Minerals and Trace Elements
Magnesium: Toxicity, Genetic diseases, Requirements, dietary sources, Micronutrient interactions
Magnesium
Like calcium, magnesium is an alkaline earth metal. Magnesium is the eighth most abundant element in the Earth’s crust. It does not occur uncombined, but is found in large deposits in the form of magnesite, dolomite, and other minerals. The metal is used in flashlight photography, flares, and pyrotechnics. It is one-third lighter than aluminum, and in alloys is essential for airplane and missile construction. Magnesium is used in producing nodular graphite in cast iron and as an additive to conventional propel-lants. The hydroxide (milk of magnesia), chloride, sulfate (Epsom salts), and citrate are used in medicine.
Magnesium was first shown to be an essential dietary component for rats in 1932 and later for humans. This essentiality is a reflection of the role that magnesium plays in the stabilization of ATP and other molecules. Since then, nutritionists have come to realize that frank magnesium deficiency is rare and that it only occurs in clinical settings as a secondary consequence of another disease. More recently, mod-erate or marginal deficiency has been proposed as a risk factor for chronic diseases such as osteoporosis, cardiovascular disease, and diabetes. These associa-tions are controversial.
Toxicity
Magnesium, when ingested as a naturally occurring substance in foods, has not been demonstrated to exert any adverse effects in people with normal renal function. However, adverse effects of excess magne-sium intake (e.g., diarrhea, nausea, abdominal cramp-ing) have been observed with intakes from nonfood sources such as various magnesium salts used for pharmacological purposes. For this reason the US Food and Nutrition Board established the tolerable UL for adolescents and adults as 350 mg of nonfood magnesium.
Genetic diseases
Several disease states are associated with magnesium deficiency, some of which have genetic roots, for example hypomagnesemia with secondary hypocal-cemia, primary hypomagnesemia with hypercalciuria, renal hypomagnesemia 2, Bartter’s syndrome, and Gitelman’s syndrome.
Primary hypomagnesemia with hypercalciuria is caused by a mutation in the paracellin-1 (PCLN1) gene on chromosome 3. PCLN1 is a component of the tight junction complex in nephrons and, therefore, has a role in renal magnesium ion reabsorption. Hypomagnesemia with secondary hypocalcemia is an autosomal recessive disorder and is determined by a mutation in a gene located on 9q12–q22.2.
Assessing status
Estimating magnesium requirements and establish-ing magnesium–disease relationships depend on accurate and specific indicators of magnesium status. Several such indicators have been described. All of these are based on measurement of the magnesium content in various body pools. Analysis of total mag-nesium in serum is often used as an indicator of mag-nesium status, although only about 1% of total body magnesium is present in ECF. It has been suggested that the concentration of ionized magnesium in serum may be a more reliable and relevant determi-nant of magnesium deficiency. In addition, intracel-lular magnesium concentration (usually measured in accessible tissues such as erythrocytes and lympho-cytes) provides a more accurate assessment of body magnesium status than does the concentration of magnesium in serum. The dietary balance approach is considered to be the best available method for estimating magnesium requirements. Although this method is a powerful research tool for the study of magnesium homeostasis, it is time, resource, and labor intensive, and these limit its application to large populations. None of the currently available proce-dures is perfect for all circumstances.
Requirements and dietary sources
In 1997, the US RDA [the nutrient intake value that is sufficient to meet the requirement of nearly all (97–98%) individuals in a life-stage and sex group] for magnesium was revised upwards for most groups. The current RDA for adult women is now 320 mg/day and for adult men is 420 mg/day. An additional value is now part of the US Food and Nutrition Board’s dietary reference intakes, the estimated average requirement (EAR; the nutrient intake value that is estimated to meet the requirement of 50% of the individuals in a life-stage and sex group). This esti-mate was set to 265 and 350 mg/day for adult women and men, respectively. This value is similar to the mean magnesium intake reported for women and men in the USA (228 and 323 mg/day). Collectively, these data suggest that most Americans are not con-suming enough magnesium in their diet and this also appears to be the case for several European popula-tions. However, while the public health relevance of this observation is currently being debated, the fact that there is not a universally accepted reliable mag-nesium status assessment tool makes it difficult to determine the actual consequence of this apparent low intake.
For those who want to increase their magnesium intake, a number of high magnesium foods and dietary practices will lead to adequate intake. Foods with a high magnesium content include whole grains, legumes, green leafy vegetables, and tofu; meat, fruits, and dairy products have an intermediate magnesium content (Table 9.4). The poorest sources of magne-sium are refined foods. Although high levels of calcium, phosphate, or fiber may lead to reduced bio-availability of magnesium, differences in bioavailabil-ity of magnesium from various food sources does not
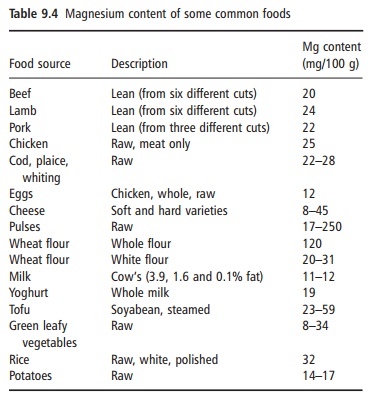
appear to be a significant barrier to achieving ade-quate magnesium status. Thus, the current recom-mendations for a healthy diet based on the food pyramid are consistent with the goals of reaching the US RDA for magnesium.
Micronutrient interactions
As mentioned above, phosphorus as phosphate, espe-cially in phytate, may decrease intestinal magnesium absorption. In general, calcium intake in the usual dietary range does not affect magnesium absorption, but calcium intakes in excess of 2.6 g have been reported to reduce magnesium balance. Magnesium intake in the usual dietary range does not appear to alter calcium balance.
Related Topics