Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Kossel-Lewis approach to Chemical Bonding
Kossel-Lewis approach to Chemical Bonding
W.Kossel laid down the following postulates to the understanding of
ionic bonding:
1.
In the periodic table, the highly
electronegative halogens and the highly electropositive alkali metals are separated
by the noble gases. Therefore one or small number of electrons are easily
gained and transferred to attain the stable noble gas configuration.
2.
The formation of a negative ion from a halogen
atom and a positive ion from an alkali metal atom is associated with the gain
and loss of an electron by the respective atoms.
3.
The negative and positive ions so formed attains
stable noble gas electronic configurations. The noble gases (with the exception
of helium which has two electrons in the outermost shell) have filled outer
shell electronic configuration of eight electrons (octet of electrons) with a
general representation ns2 np6.
4.
The negative and positive ions are bonded and
stabilised by force of electrostatic attraction.
Kossel's postulates provide the basis for the
modern concepts on electron transfer between atoms which results in ionic or
electrovalent bonding.
For
example, formation of NaCl molecule from sodium and chlorine atoms can be
considered to take place according to Kossel's theory by an electron transfer
as:
(i) Na ( [Ne]
3s1) -- loss of e --- > Na+ + e ( [Ne])
where [Ne] = electronic configuration of Neon
(ii) Cl +
e ([Ne]3s2 3p5 ) - gain of e -- > Cl-1([Ar])
[Ar] = electronic
configuration of Argon
(iii) Na++Cl-
-- electrostatic attraction -- > NaCl(or)Na+ Cl-
NaCl is an electrovalent or ionic compound made
up of sodium ions and chloride ions. The bonding in NaCl is termed as
electrovalent or ionic bonding. Sodium atom loses an electron to attain Neon configuration
and also attains a positive charge. Chlorine atom receives the electron to
attain the Argon configuration and also becomes a negatively charged ion. The
coulombic or electrostatic attraction between Na+ and Cl-
ions result in NaCl formation.
Similarly formation of MgO may be shown to occur by the transfer of two
electrons.
The bonding in MgO is also electrovalent or
ionic and the electrostatic forces of attraction binds Mg2+ ions
with O2- ions. Thus, "the binding forces existing as a result
of electrostatic attraction between the positive and negative ions", is
termed as electrovalent or ionic bond. The electrovalency is
considered as equal to the number of charges on an ion. Thus magnesium has
positive electrovalency of two while chlorine has negative electrovalency of
one.
The valence electron transfer theory could not
explain the bonding in molecules like H2, O2, Cl2 etc., and in other organic
molecules that have ions.
G.N.Lewis, proposed the octet rule to explain
the valence electron sharing between atoms that resulted in a bonding type with
the atoms attaining noble gas electronic configuration. The statement is :
"a bond is formed between two atoms by mutual sharing of pairs of
electrons to attain a stable outer-octet of electrons for each atom involved in
bonding". This type of valence electron sharing between atoms is termed as
covalent bonding. Generally homonuclear diatomics possess covalent bonds.
It is assumed that the atom consists of a `Kernel' which is made up of a
nucleus plus the inner shell electrons. The Kernel is enveloped by the outer
shells that could accommodate a maximum of eight electrons. The eight
outershell electrons are termed as octet of electrons and represents a stable
electronic configuration. Atoms achieve the stable outer octet when they are
involved in chemical bonding.
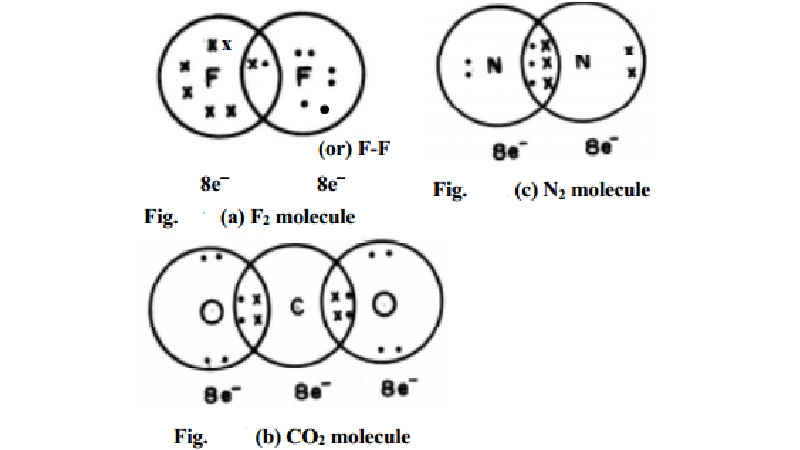
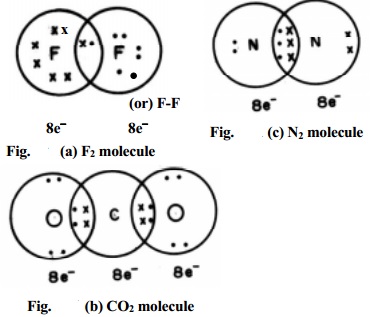
In case of molecules like F2, Cl2, H2 etc., the bond is formed by the
sharing of a pair of electrons between the atoms. For example, consider the
formation of a fluorine molecule (F2). The atom has electronic
configuration. [He]2s2 3s2 3p5 which is having
one electron less than the electronic configuration of Neon. In the fluorine
molecule, each atom
contributes one electron to the shared pair of the bond of the F2
molecule. In this process, both the fluorine atoms attain the outershell octet
of a noble gas (Argon) (Fig. (a)). Dots (·) represent electrons. Such structures are called as Lewis dot
structures.
Lewis dot structures can be written for combining of like or different
atoms following the conditions mentioned below :
1.
Each bond is the result of sharing of an
electron pair between the atoms comprising the bond.
2. Each combining atom contributes one electron to the shared pair.
3. The combining atoms attain the outer filled shells of the noble gas
configuration.
If the two atoms share a pair of electrons, a single bond is said to be
formed and if two pairs of electrons are shared a double bond is said to be
formed etc. All the bonds formed from sharing of electrons are called as
covalent bonds.
In carbon dioxide (CO2) two double bonds are seen at the
centre carbon atom which is linked to each oxygen atom by a double bond. The
carbon and the two oxygen atoms attain the Neon electronic configuration.
When the two combining atoms share three electron pairs as in N2
molecule, a triple bond is said to be formed. Each of the Nitrogen atom shares
3 pairs of electrons to attain neon gas electronic configuration.
Related Topics