Discovery, Production, Characteristic, Applications, Example Solved Problem, X-ray spectra - X-Rays | 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Chapter: 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
X-Rays
X – RAYS
Introduction
Quantum theory of radiation explains
photoelectric effect in which the electrons are emitted due to the incidence of
photons and the energy is transferred from photons to the electrons.
Immediately, a question arises: Is the reverse process also possible?
This means that whether electron
kinetic energy can be transformed into photon energy or not. The phenomenon
which answers this question has already been discovered, even before Planck’s
quantum theory of radiation.
Discovery of x-rays
Wilhelm Roentgen in 1895 discovered
that whenever fast moving electrons fall on certain materials, a highly
penetrating radiation is emitted. Since their origin was not known at that
time, they were called x-rays.
X-rays are electromagnetic waves of
short wavelength ranging from 0.1 to 100Ă….
They travel along straight lines with the velocity of light and are not
affected by electric and magnetic fields. X-ray photons are highly energetic
because of its high frequency or short wavelength. Therefore, they can pass
through materials which are opaque to visible light.
The quality of x-rays is measured in
terms of their penetrating power which depends on the velocity with which the
electrons strike the target material and the atomic number of target material. The
intensity of x-rays is dependent on the number of electrons striking the
target.
Production of x-rays
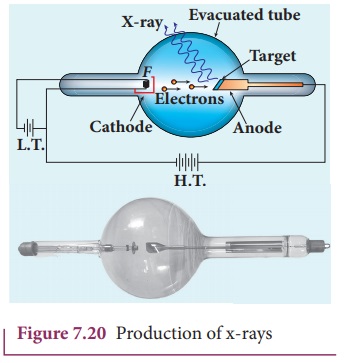
X-rays are produced in x-ray tube
which is essentially a discharge tube as shown in Figure 7.20. A tungsten
filament F is heated to incandescence
by a battery. As a result, electrons are emitted from it by thermionic
emission.
The electrons are accelerated to
high speeds by the voltage applied between the filament F and the anode. The target materials like tungsten, molybdenum are
embedded in the face of the solid copper anode. The face of the target is
inclined at an angle with respect to the electron beam so that x-rays can leave
the tube through its side.
When high-speed electrons strike the
target, they are decelerated suddenly and lose their kinetic energy. As a
result, x-ray photons are produced. Since most of the kinetic energy of the
bombarding electrons gets converted into heat, targets made of
high-melting-point metals and a cooling system are usually employed.
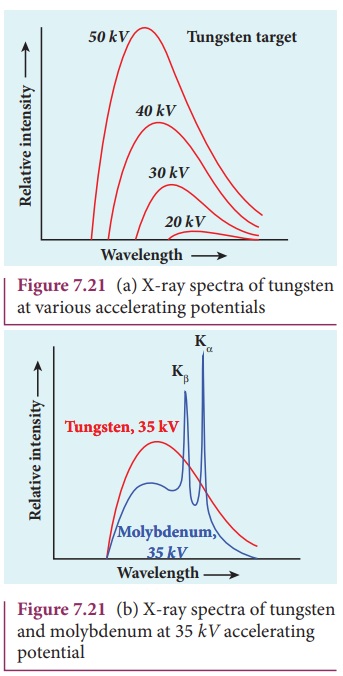
X-ray spectra
X-rays are produced when fast moving
electrons strikethemetal target. The intensity of the x-rays when plotted
against its wavelength gives a curve called x-ray spectrum (Figure 7.21(a) and (b)). X-ray spectra consist of
two parts: a continuous spectrum and a series of peaks superimposed on it.
The continuous spectrum consists of radiations of all possible wavelengths
with a certain minimum wavelength λ0 which depends on the voltage
across the electrodes. The peaks are characteristics of the material of the
target and hence they are called characteristic
spectrum. Figure 7.21(a) depicts the x-ray spectra of tungsten at various
accelerating voltages and Figure 7.21(b) shows the x-ray spectra of tungsten
and molybdenum at a particular accelerating voltage.
Though classical electromagnetic
theory suggests the emission of radiations from accelerating electrons, it
could not explain two features exhibited by x-ray spectra.
These features are given below.
(i) For a given
accelerating voltage, the lower limit for the wavelength of continuous x-ray
spectra is same for all targets. This minimum wavelength is called cut-off
wavelength.
(ii) The intensity of x-rays is
significantly increased at certain well-defined wavelengths as shown in the case
of characteristic x-ray spectra for molybdenum (Figure 7.21(b)).
But these two features could be
explained on the basis of photon theory of radiation.
Continuous x-ray spectra
When a fast moving electron
penetrates and approaches a target nucleus, the interaction between the
electron and the nucleus either accelerates or decelerates it which results in
a change of path of the electron. The radiation produced from such decelerating
electron is called Bremsstrahlung or
braking radiation (Figure 7.22).
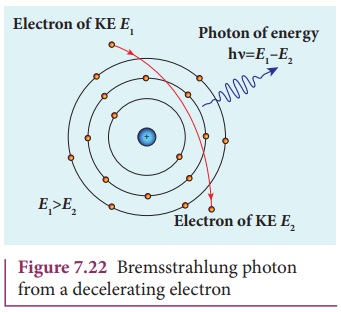
The energy of the photon emitted is
equal to the loss of kinetic energy of the electron. Since an electron may lose
part or all of its energy to the photon, the photons are emitted with all possible
energies (or frequencies). The continuous x-ray spectrum is due to such
radiations.
When an electron gives up all its
energy, then the photon is emitted with highest frequency
ν0 (or lowest wavelength λ0
). The initial kinetic energy of an electron is given by eV where V is the
accelerating voltage. Therefore, we have
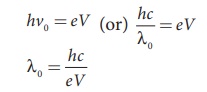
where λ0 is the cut-off
wavelength. Substituting the known values in the above equation, we get
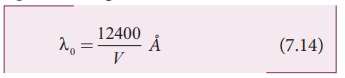
The relation given by equation
(7.14) is known as the Duane – Hunt formula.
The value of λ0 depends
only on the accelerating potential and is same for all targets. This is in good
agreement with the experimental results. Thus, the production of continuous
x-ray spectrum and the origin of cut – off wavelength can be explained on the
basis of photon theory of radiation.
Characteristic x – ray spectra:
X – ray spectra show some narrow
peaks at some well – defined wavelengths when the target is hit by fast
electrons. The line spectrum showing these peaks is called characteristic x – ray spectrum. This x – ray spectrum is due to
the electronic transitions within the atoms.
When an energetic electron
penetrates into the target atom and removes some of the K-shell electrons. Then the electrons from outer orbits jump to
fill up the vacancy so created in the K-shell.
During the downward transition, the energy difference between the levels is
given out in the form of x– ray photon of definite wavelength. Such
wavelengths, characteristic of the target, constitute the line spectrum.
From the Figure 7.23, it is evident
that K-series of lines in the x-ray
spectrum of an element arises due to the electronic transitions from L, M,
N, . . levels to the K-level. Similarly, the longer
wavelength L-series originates when an
L-electron is knocked out of the atom
and the corresponding vacancy is filled by the electronic transitions from M, N,
O,... and so on.
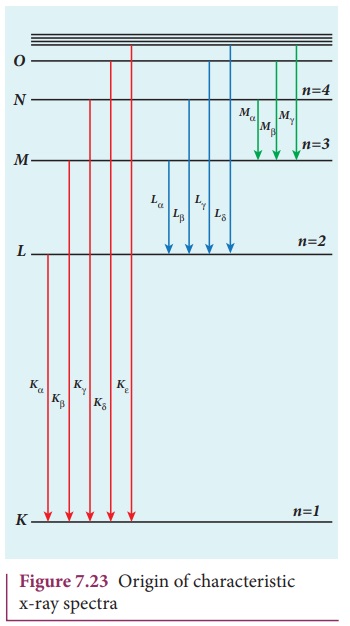
The Kα and Kβ of the K-series
of molybdenum are shown by the two peaks in its
x-ray spectrum in Figure 7.21(b).
Applications of x-rays:
X-rays are being used in many
fields.
Let us list a few of them.
i) Medical diagnosis
X-rays can pass through flesh more
easily than through bones. Thus an x-ray radiograph containing a deep shadow of
the bones and a light shadow of the flesh may be obtained. X-ray radiographs
are used to detect fractures, foreign bodies, diseased organs etc.
ii) Medical therapy
Since x-rays can kill diseased
tissues, they are employed to cure skin diseases, malignant tumours etc.
iii) Industry
X-rays are used to check for flaws
in welded joints, motor tyres, tennis balls and wood. At the custom post, they
are used for detection of contraband goods.
iv) Scientific research
X-ray diffraction is important tool
to study the structure of the crystalline materials – that is, the arrangement
of atoms and molecules in crystals.
EXAMPLE 7.9
Calculate the cut-off wavelength and
cut- off frequency of x-rays from an x –ray tube of accelerating potential
20,000 V.
Solution
The cut-off wavelength of the
characteristic x-rays is
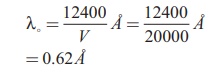
= 0.62 Ă…
The corresponding frequency is
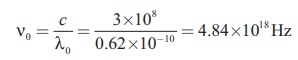
Related Topics