Dual Nature of Radiation and Matter | Physics - Multiple Choice Questions | 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Chapter: 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Multiple Choice Questions
I Multiple Choice Questions
1. The wavelength ╬╗e of an electron and ╬╗p of a photon of same energy E are related by
a. ╬╗p ŌłØ ╬╗e
b. ╬╗p ŌłØ ŌłÜ╬╗e
c. ╬╗p ŌłØ 1/ŌłÜ╬╗e
d. ╬╗p ŌłØ ╬╗2e
Answer: d)
Solution:
de
Broglie wavelength for an electron
╬╗e=
h / ŌłÜ(2mE) ;
╬╗e
ŌłØ 1/ŌłÜE ;
╬╗e2
ŌłØ 1/E ------------- (1)
For
photon
╬╗p=
hc / E ;
╬╗p
ŌłØ 1/E ------------- (2)
From
equation (1) and (2), we have So,
╬╗p ŌłØ ╬╗e2
2. In an electron microscope, the electrons are accelerated by a voltage of 14 kV. If the voltage is changed to 224 kV, then the de Broglie wavelength associated with the electrons would
a. increase by 2 times
b. decrease by 2 times
c. decrease by 4 times
d. increase by 4 times
Answer: c)
Solution:
deBroglie
wavelength ╬╗ ŌłØ 1/ŌłÜV
╬╗1/╬╗2 = ŌłÜ(V2/V1)
= ŌłÜ(224/14) = ŌłÜ16 = 4
╬╗1
= 4╬╗2
╬╗2
= ╬╗1/4
3. A particle of mass 3 ├Ś 10ŌĆō6 g has the same wavelength as an electron moving with a velocity 6├Ś106 m sŌłÆ1 . The velocity of the particle is
a. 1.82├Ś10ŌłÆ18 m sŌłÆ1
b. 9├Ś10ŌłÆ2 m sŌłÆ1
c. 3├Ś10ŌłÆ31m sŌłÆ1
d. 1.82├Ś10ŌłÆ15 m sŌłÆ1
Answer: d)
Solution:
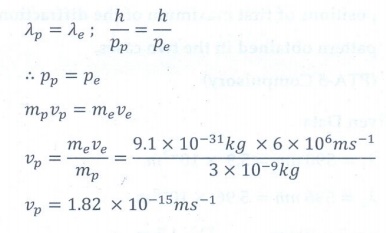
4. When a metallic surface is illuminated with radiation of wavelength ╬╗, the stopping potential is V. If the same surface is illuminated with radiation of wavelength 2╬╗, the stopping potential is V/4. The threshold wavelength for the metallic surface is
a. 4╬╗
b. 5╬╗
c. 5/2 ╬╗
d. 3╬╗
Answer: d)
Solution:
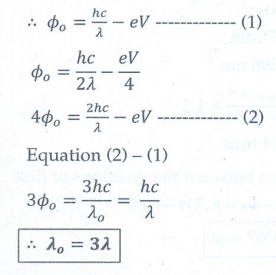
5. If a light of wavelength 330 nm is incident on a metal with work function 3.55 eV, the electrons are emitted. Then the wavelength of the emitted electron is (Take h = 6.6 ├Ś 10ŌĆō34 Js)
a. < 2.75├Ś10ŌłÆ9 m
b. Ōēź 2.75├Ś10ŌłÆ9 m
c. Ōēż 2.75├Ś10ŌłÆ12 m
d. < 2.5├Ś10ŌłÆ10 m
Answer: b)
Solution:
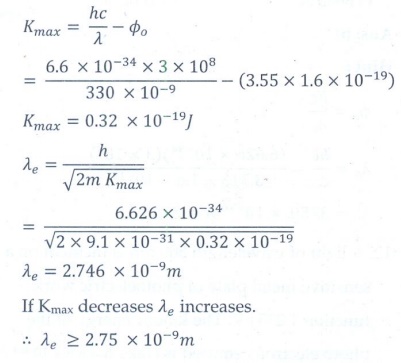
6. A photoelectric surface is illuminated successively by monochromatic light of wavelength ╬╗ and ╬╗/2. If the maximum kinetic energy of the emitted photoelectrons in the second case is 3 times that in the first case, the work function at the surface of material is
a) hc/╬╗
b) 2hc/ ╬╗
c) hc/3╬╗
d) hc/2╬╗
Answer: d)
Solution:

7. In photoelectric emission, a radiation whose frequency is 4 times threshold frequency of a certain metal is incident on the metal. Then the maximum possible velocity of the emitted electron will be
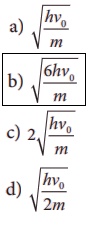
Answer: b)
Solution:
From
Enistein's equation

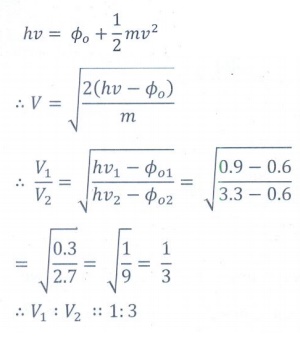
8. Two radiations with photon energies 0.9 eV and 3.3 eV respectively are falling on a metallic surface successively. If the work function of the metal is 0.6 eV, then the ratio of maximum speeds of emitted electrons will be
a) 1:4
b) 1:3
c) 1:1
d) 1:9
Answer: b)
Solution:
9. A light source of wavelength 520 nm emits 1.04 ├Ś 1015 photons per second while the second source of 460 nm produces 1.38 ├Ś 1015 photons per second. Then the ratio of power of second source to that of first source is
a) 1.00
b) 1.02
c) 1.5
d) 0.98
Answer: c)
Solution:
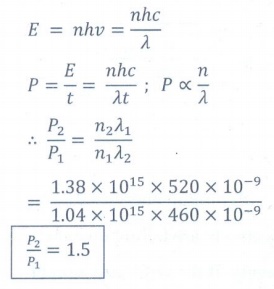
10. The mean wavelength of light from sun is taken to be 550 nm and its mean power is 3.8 ├Ś 1026W. The number of photons received by the human eye per second on the average from sunlight is of the order of
a) 1045
b) 1042
c) 1054
d) 1051
Answer: a)
Solution:
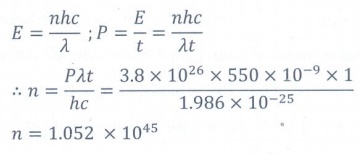
11. The threshold wavelength for a metal surface whose photoelectric work function is 3.313 eV is
a) 4125├ģ
b) 3750 ├ģ
c) 6000 ├ģ
d) 2062.5├ģ
Answer: b)
Solution:
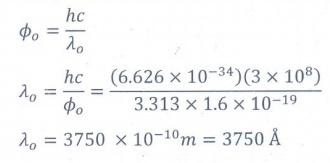
12. A light of wavelength 500 nm is incident on a sensitive plate of photoelectric work function 1.235 eV. The kinetic energy of the photo electrons emitted is be (Take h = 6.6 ├Ś 10ŌĆō34 Js)
a) 0.58 eV
b) 2.48 eV
c) 1.24 eV
d) 1.16 eV
Answer: c)
Solution:
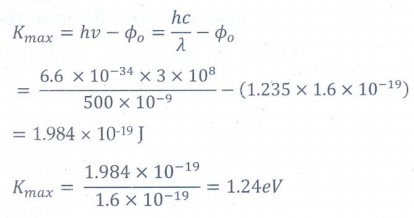
13. Photons of wavelength ╬╗ are incident on a metal. The most energetic electrons ejected from the metal are bent into a circular arc of radius R by a perpendicular magnetic field having magnitude B. The work function of the metal is
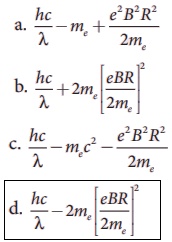
Answer: option d
Answer: d)
Solution:
Centripetal
force (Fc.p) = Magnetic force (FB)
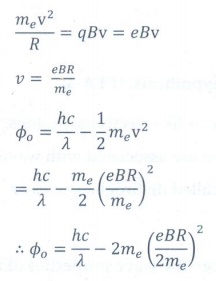
14. The work functions for metals A, B and C are 1.92 eV, 2.0 eV and 5.0 eV respectively. The metals which will emit photoelectrons for a radiation of wavelength 4100 ├ģ is/are
a. A only
b. both A and B
c. all these metals
d. none
Answer: b)
Solution:
The energy of the light with the wavelength 4100
├Ś 10-10 m
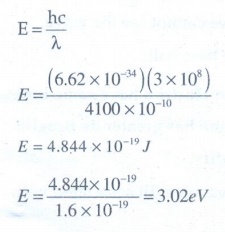
Both
(A) and (B) emits the electrons, because its work function is less than 3.02eV.
15. Emission of electrons by the absorption of heat energy is called _______ emission.
a. photoelectric
b. field
c. thermionic
d. secondary
Answer: c)
Answers
1. d 2. c 3. d 4. d 5. b 6. d 7. b 8. b 9. c 10. a 11. b 12. c 13. d 14. b 15. C
Related Topics