Wave nature of particles | Physics - De Broglie wave length of electrons | 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Chapter: 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
De Broglie wave length of electrons
De Broglie wave:
The wave–particle duality of
radiation was extended to matter by a French physicist Louis de Broglie
(pronounced as de Broy) in 1924.
Greatly influenced by the symmetry
in nature, de Broglie suggested that if radiation like light can act as
particles at times, then matter particles like electrons should also act as
waves at times.
According to de Broglie hypothesis, all
matter particles like electrons, protons, neutrons in motion are associated
with waves. These waves are called de Broglie waves or matter waves.
De Broglie wave length:
The momentum of photon of frequency ν is given by
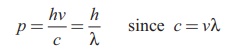
The wavelength of a photon in terms
of its momentum is

According to de Broglie, the above
equation is completely a general one and this is applicable to material
particles as well. Therefore, for a particle of mass m travelling with speed Ď…, the wavelength is given by
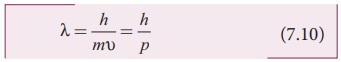
This wavelength of the matter waves
is known as de Broglie wavelength.
This equation relates the wave character (the wave length λ) and the particle
character (the momentum p) through
Planck’s constant.
De Broglie wave length of electrons:
An electron of mass m is accelerated through a potential
difference of V volt. The kinetic
energy acquired by the electron is given by
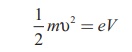
Therefore, the speed Ď… of the
electron is

Hence, the de Broglie wavelength of
the electron is
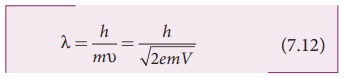
Substituting the known values in the
above equation, we get
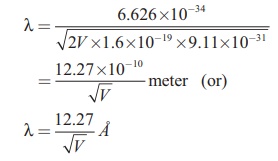
For example, if an electron is
accelerated through a potential difference of 100V, then its de Broglie wavelength is 1.227 Ă….
Since the kinetic energy of the
electron, K = eV, then the de Broglie wavelength associated with electron can be
also written as

Related Topics