Photo Electric Effect | Physics - Particle nature of light: EinsteinŌĆÖs explanation | 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Chapter: 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Particle nature of light: EinsteinŌĆÖs explanation
PHOTO ELECTRIC EFFECT
Particle nature of light: EinsteinŌĆÖs explanation
Einstein extended PlanckŌĆÖs quantum
concept to explain the photoelectric effect in 1905. According to Einstein, the
energy in light is not spread out over wavefronts but is concentrated in small
packets or energy quanta. Therefore, light (or any other electromagnetic waves)
of frequency v from any source can be
considered as a stream of quanta and the energy of each light quantum is given
by E = h╬Į.
He also proposed that a quantum of
light
has linear momentum and the magnitude of that linear momentum is p = h╬Į / c . The individual
light quantum of definite energy and momentum
can be associated with a particle. The light
quantum can behave as a particle and this is called photon. Therefore, photon
is nothing but particle manifestation of light.
Characteristics of photons:
According to particle nature of
light, photons are the basic constituents of any radiation and possess the
following characteristic properties:
i) The photons of light of frequency ╬Į and wavelength ╬╗ will have energy, given by E = hv = hc/╬╗ .
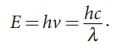
ii) The energy of a photon is
determined by the frequency of the radiation and not by its intensity and the
intensity has no relation with the energy of the individual photons in the
beam.
iii) The photons travel with the velocity of light and its momentum is given by p = h/╬╗ = hv/c
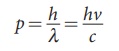
iv) Since photons are electrically
neutral, they are unaffected by electric and magnetic fields.
v) When a photon interacts with
matter (photon-electron collision), the total energy, total linear momentum and
angular momentum are conserved. Since photon may be absorbed or a new photon
may be produced in such interactions, the number of photons may not be
conserved.
According to quantum concept,
intensity of light of given wavelength is defined as the number of energy
quanta or photons incident per unit area per unit time, with each photon having
same energy. The unit is WmŌĆō2.
EinsteinŌĆÖs explanation of photoelectric equation
![]() When
a photon of energy h╬Į is incident on
a metal surface, it is completely absorbed by a single electron and the
electron is ejected. In this process, a part of the photon energy is used for
the ejection of the electrons from the metal surface (photoelectric work
function ŽĢ0 ) and the remaining energy as the
kinetic energy of the ejected electron. From the law of conservation of energy,
When
a photon of energy h╬Į is incident on
a metal surface, it is completely absorbed by a single electron and the
electron is ejected. In this process, a part of the photon energy is used for
the ejection of the electrons from the metal surface (photoelectric work
function ŽĢ0 ) and the remaining energy as the
kinetic energy of the ejected electron. From the law of conservation of energy,
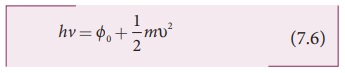
where m is the mass of the electron and Žģ its
velocity. This is shown in Figure 7.13(a).
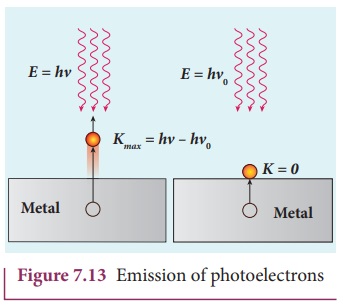
If we reduce the frequency of the
incident light, the speed or kinetic energy of photo electrons is also reduced.
At some frequency ╬Į0 of
incident radiation, the photo electrons are ejected with almost zero kinetic
energy (Figure 7.13(b)). Then the equation (7.6) becomes
hv0 = ŽĢ0
where ╬Į0 is the threshold frequency. By rewriting the equation
(7.6), we get
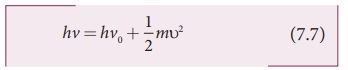
The equation (7.7) is known as EinsteinŌĆÖs photoelectric equation.
If the electron does not lose energy
by internal collisions, then it is emitted with maximum kinetic energy Kmax. Then
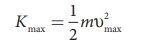
where Žģmax is the maximum
velocity of the electron ejected. The equation (7.6) is rearranged as follows:
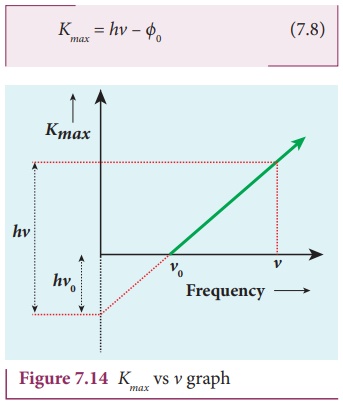
A graph between maximum kinetic
energy Kmax of the photoelectron and frequency ╬Į of the incident light is a straight line as shown in Figure
7.14. The slope of the line is h and its y-intercept is ŌĆōŽĢ0.
EinsteinŌĆÖs equation was
experimentally verified by R.A. Millikan. He drew Kmax versus ╬Į graph
for many metals (cesium, potassium, sodium and lithium) as shown in Figure 7.15
and found that the slope is independent of the metals.
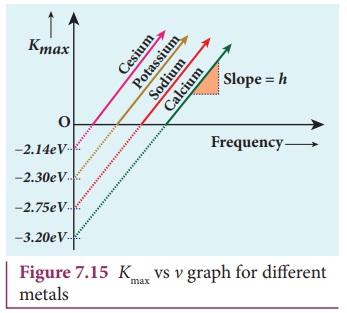
Millikan also calculated the value
of PlanckŌĆÖs constant (h=6.626├Ś10ŌĆō34
Js) and work function of many metals
(Cs, K, Na, Ca); these values are in agreement with the theoretical prediction.
Explanation for the photoelectric effect:
The experimentally observed facts of
photoelectric effect can be explained with the help of EinsteinŌĆÖs photoelectric
equation.
i) As each incident photon liberates
one electron, then the increase of intensity of the light (the number of
photons per unit area per unit time) increases the number of electrons emitted
thereby increasing the photocurrent. The same has been experimentally observed.
ii) From Kmax = hv ŌĆō ŽĢ0, it is
evident that Kmax is
proportional to the frequency of the light and is independent of intensity of the
light.
iii) As given in equation (7.7),
there must be minimum energy (equal to the work function of the metal) for
incident photons to liberate electrons from the metal surface. Below which,
emission of electrons is not possible. Correspondingly, there exists minimum
frequency called threshold frequency below which there is no photoelectric
emission.
iv) According to quantum concept,
the transfer of photon energy to the electrons is instantaneous so that there
is no time lag between incidence of photons and ejection of electrons.
Thus, the photoelectric effect is
explained on the basis of quantum concept of light.
The nature of light: wave - particle duality
We have learnt that wave nature of
light explains phenomena such as interference, diffraction and polarization.
Certain phenomena like black body radiation, photoelectric effect can be
explained by assigning particle nature to light. Therefore, both theories have
enough experimental evidences.
In the past, many scientific
theories have been either revised or discarded when they contradicted with new
experimental results.
Here, two different theories are
needed to answer the question: what is nature of light?
It is therefore concluded that light
possesses dual nature, that of both particle and wave. It behaves like a wave
at some circumstances and it behaves like a particle at some other circumstances.
In other words, light behaves as a
wave during its propagation and behaves as a particle during its interaction
with matter. Both theories are necessary for complete description of physical
phenomena. Hence, the wave nature and quantum nature complement each other.
A reader may find it difficult to understand
how light can be both a wave and a stream of particle. This is the case even
for great scientist like Albert Einstein.
Einstein once wrote a letter to his
friend Michel Besso in 1954 expressing his frustration:
ŌĆ£All these fifty years of conscious
brooding have brought me no closer to answer the question, ŌĆśWhat are light
quanta?ŌĆÖ Of course today everyone thinks he knows the answer, but he is
deluding himself ŌĆØ.
Related Topics