Physics - Photo Electric Effect: Example Numerical Problems | 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Chapter: 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Photo Electric Effect: Example Numerical Problems
PHOTO ELECTRIC EFFECT
EXAMPLE 7.2
A radiationof wavelength 300 nm is incident on a silver surface. Will
photoelectrons be observed?
Solution:
Energy of the incident photon is
E = hv = hc/╬╗ (in joules)
E = hc/╬╗e (in eV)
Substituting the known values, we
get
E = 6.626├Ś10ŌłÆ34 ├Ś3├Ś108
/ 300├Ś10ŌłÆ9 ├Ś1.6├Ś10ŌłÆ19
E = 4.14 eV
From Table 7.1, the work function of
silver = 4.7 eV. Since the energy of the
incident photon is less than the work function of silver, photoelectrons are not
observed in this case.
EXAMPLE 7.3
When light of wavelength 2200├ģ falls on Cu, photo electrons are
emitted from it. Find (i) the threshold wavelength and (ii) the stopping potential. Given: the work function for Cu
is ŽĢ0 = 4.65 eV.
Solution
i) The threshold wavelength is given
by
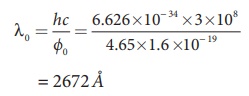
= 2672 ├ģ
ii) Energy of the photon of
wavelength 2200 ├ģ is
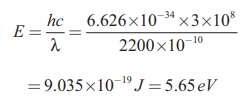
= 9.035├Ś10ŌłÆ19 J = 5.65 eV
We know that kinetic energy of
fastest photo electron is
Kmax = hv ŌĆō ŽĢ0 = 5.65 ŌĆō 4.65
= 1 eV
From equation (7.3), Kmax = eV0
V0 = Kmax/e 1├Ś1.6├Ś10ŌłÆ19
/ 1.6├Ś10ŌłÆ19
Therefore, stopping potential = 1V
EXAMPLE 7.4
The work function of potassium is
2.30 eV. UV light of wavelength 3000 ├ģ and intensity 2 Wm-2 is incident on the potassium surface. i) Determine
the maximum kinetic energy of the photo electrons ii) If 40% of incident photons produce photo
electrons, how many electrons are emitted per second if the area of the
potassium surface is 2 cm2
?
Solution
i) The energy of the photon is
E = hc/╬╗
= 6.626├Ś10ŌłÆ34 ├Ś 3├Ś108 / 3000├Ś10ŌłÆ10
E = 6.626├Ś10ŌłÆ19 J = 4.14 eV
Maximum KE of the photoelectrons is
Kmax = hv ŌĆō ŽĢ0 = 4.14 ŌĆō 2.30 = 1.84 eV
ii) The number of photons reaching
the surface per second is
np = P/E ├Ś
A
= [ 2 / 6.626├Ś10ŌłÆ19 ] ├Ś [2├Ś10ŌłÆ4]
= 6.04├Ś1014 photons / sec
The rate of emission of
photoelectrons is
= (0.40)np = 0.4├Ś6.04├Ś1014
= 2.415┬┤1014 photoelectrons
/ sec
EXAMPLE 7.5
Light of wavelength 390 nm is directed at a metal electrode. To
find the energy of electrons ejected, an opposing potential difference is established
between it and another electrode. The current of photoelectrons from one to the
other is stopped completely when the potential difference is 1.10 V. Determine i) the work function of the
metal and ii) the maximum wavelength of light that can eject electrons from
this metal.
Solution
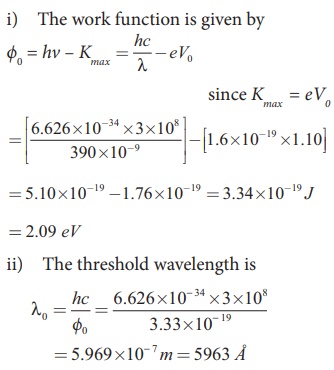
Related Topics