Chapter: 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Wave nature of particles: Example Solved Numerical Problems
Wave nature of particles
EXAMPLE 7.6
Calculate the momentum and the de
Broglie wavelength in the following cases:
i) an electron with kinetic energy 2
eV.
ii) a bullet of 50 g fired from
rifle with a speed of 200 m/s
iii) a 4000 kg car moving along the
highways at 50 m/s
Hence show that the wave nature of
matter is important at the atomic level but is not really relevant at
macroscopic level.
Solution:
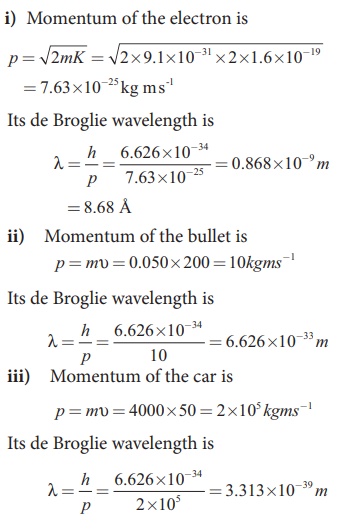
From these calculations, we notice
that electron has significant value of de Broglie wavelength (Ōēł10ŌĆō9m which can be measured from difraction
studies) but bullet and car have negligibly small de Broglie wavelengths
associated with them (Ōēł10ŌĆō33m and
10ŌĆō39m respectively, which
are not measurable by any experiment). This implies that the wave nature of
matter is important at the atomic level but it is not really relevant at the
macroscopic level.
EXAMPLE 7.7
Find the de Broglie wavelength
associated with an alpha particle which is accelerated through a potential
difference of 400 V. Given that the
mass of the proton is 1.67 ├Ś 10ŌĆō27 kg.
Solution
An alpha particle contains 2 protons
and 2 neutrons. Therefore, the mass M of
the alpha particle is 4 times that of a proton (mp) (or a neutron) and its charge q is twice that of a proton (+e).
The de Broglie wavelength associated
with it is
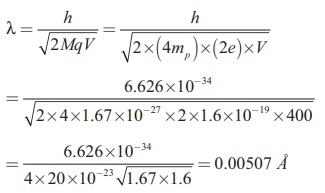
EXAMPLE 7.8
A proton and an electron have same
de Broglie wavelength. Which of them moves faster and which possesses more
kinetic energy?
Solution
We know that ╬╗ = h/ ŌłÜ(2mK)
Since proton and electron have same
de Broglie wavelength, we get
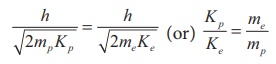
Since me < mp
, Kp < Ke , the electron has more
kinetic energy than the proton.
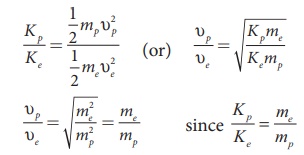
Since me < mp
, Žģp < Žģe , the electron moves faster
than the proton.
Related Topics