Photo Electric Effect | Physics - Hertz, Hallwachs and LenardŌĆÖs observation | 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Chapter: 12th Physics : UNIT 8 : Dual Nature of Radiation and Matter
Hertz, Hallwachs and LenardŌĆÖs observation
PHOTO ELECTRIC EFFECT
Hertz, Hallwachs and LenardŌĆÖs observation
Hertz observation
MaxwellŌĆÖs theory of electromagnetism
predicted the existence of electromagnetic waves and concluded that light
itself is just an electromagnetic wave. Then the experimentalists tried to
generate and detect electromagnetic waves through various experiments.
In 1887, Heinrich Hertz first became
successful in generating and detecting electromagnetic wave with his high
voltage induction coil to cause a spark discharge between two metallic spheres
(we have learnt this in Unit 5 of XII standard physics). When a spark is
formed, the charges will oscillate back and forth rapidly and the
electromagnetic waves are produced.
The electromagnetic waves thus
produced were detected by a detector that has a copper wire bent in the shape
of a circle. Although the detection of waves is successful, there is a problem
in observing the tiny spark produced in the detector.
In order to improve the visibility of
the spark, Hertz made many attempts and finally noticed an important thing that
small detector spark became more vigorous when it was exposed to ultraviolet
light.
The reason for this behaviour of the
spark was not known at that time. Later it was found that it is due to the
photoelectric emission. Whenever ultraviolet light is incident on the metallic
sphere, the electrons on the outer surface are emitted which caused the spark
to be more vigorous.
It is interesting
to note that the experiment of Hertz confirmed that light is an electromagnetic
wave. But the same experiment also produced the first evidence for particle
nature of light.
HallwachsŌĆÖ observation
In 1888, Wilhelm Hallwachs, a German
physicist, confirmed that the strange behaviour of the spark is due to the
action of ultraviolet light with his simple experiment.
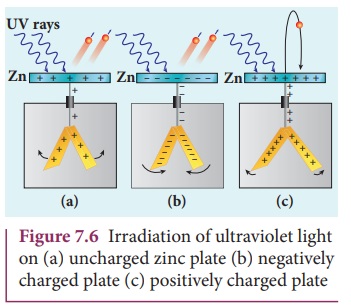
A clean circular plate of zinc is
mounted on an insulating stand and is attached to a gold leaf electroscope by a
wire. When the uncharged zinc plate is irradiated by ultraviolet light from an
arc lamp, it becomes positively charged and the leaves will open as shown in
Figure 7.6(a).
Further, if the negatively charged
zinc plate is exposed to ultraviolet light, the leaves will close as the
charges leaked away quickly (Figure 7.6(b)). If the plate is positively
charged, it becomes more positive upon UV rays irradiation and the leaves will
open further (Figure 7.6(c)). From these observations, it was concluded that
negatively charged electrons were emitted from the zinc plate under the action
of ultraviolet light.
LenardŌĆÖs observation
![]() In
1902, Lenard studied this electron emission phenomenon in detail. His simple
experimental setup is as shown in Figure 7.7.
The apparatus consists of two metallic plates A and C placed in an
evacuated quartz bulb. The galvanometer G
and battery B are connected in
the circuit.
In
1902, Lenard studied this electron emission phenomenon in detail. His simple
experimental setup is as shown in Figure 7.7.
The apparatus consists of two metallic plates A and C placed in an
evacuated quartz bulb. The galvanometer G
and battery B are connected in
the circuit.
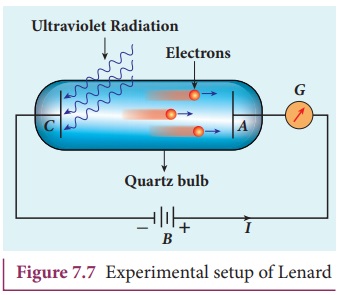
When ultraviolet light is incident
on the negative plate C, an electric
current flows in the circuit that is indicated by the deflection in the
galvanometer. On other hand, if the positive plate is irradiated by the
ultraviolet light, no current is observed in the circuit.
From these observations, it is
concluded that when ultraviolet light falls on the negative plate, electrons
are ejected from it which are attracted by the positive plate A. On reaching the positive plate
through the evacuated bulb, the circuit is completed and the current flows in
it. Thus, the ultraviolet light falling on the negative plate causes the
electron emission from the surface of the plate.
Photoelectric effect
The ejection of electrons from a metal
plate when illuminated by light or any other electromagnetic radiation of
suitable wavelength (or frequency) is called photoelectric effect. Although these electrons are not different
from all other electrons, it is customary to call them as photoelectrons and the corresponding current as photoelectric current or photo current.
Metals like cadmium, zinc, magnesium
etc show photoelectric emission for ultraviolet light while some alkali metals
lithium, sodium, caesium respond well even to larger wavelength radiation like
visible light. The materials which eject photoelectrons upon irradiation of
electromagnetic wave of suitable wavelength are called photosensitive materials.
Related Topics