Chapter: 11 th 12th std standard Bio Botany plant tree Biology Higher secondary school College Notes
Theories explaining the mechanism of enzyme action
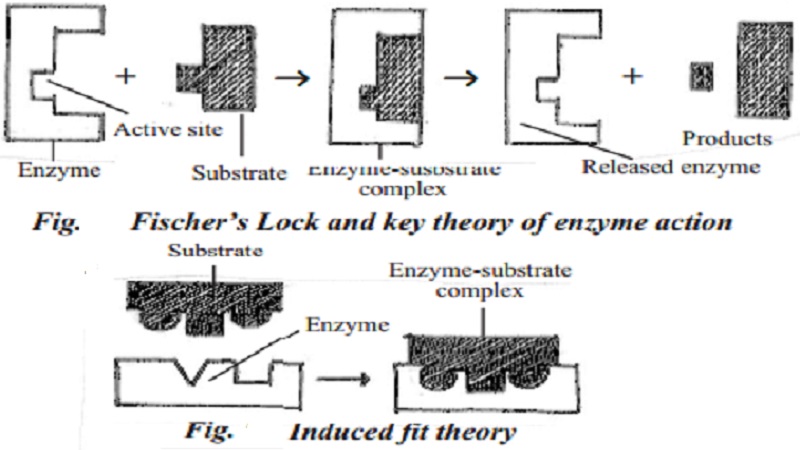
Theories explaining the mechanism of enzyme action
Two theories have been proposed to explain the mechanism and enzyme action. They are Fischer's Lock and key theory and Koshland's induced fit theory.
Fischer's Lock and key theory
Lock and key theory was proposed by Fisher. According to this theory, first a physical contact is made between the enzyme and the substrate. As only a specific key fits in a particular lock to open it, a specific substrate combines with the active site of specific enzyme. This combination leads to the production of enzyme - substrate complex. Then the enzyme acts on the substrate and changes it into products. After the reaction is over, enzyme is released from the enzyme - substrate complex and is ready to bind with another molecule of the substrate for further action. The cyclic reaction is summarized by the equation
E + S -><-[ES]-><-PS
( where, E - enzyme, S - substrate and P - product)
When a dissimilar substrate approaches the enzyme, it cannot combine with the active site of the enzyme, as a wrong key cannot open the lock. Thus, the enzyme action is inhibited.
Koshland's induced fit theory
Induced fit theory was pro-posed by Koshland. Proteins are not rigid. The substrate induces the enzyme to adjust its shape leading to the formation of enzyme sub-strate complex. Then, the enzyme acts on substrate and forms products. Many enzymes function in this way.
Mechanism of enzyme action
In a biochemical reaction, there is an energy barrier between the reactants and the products. Only those molecules which possess a certain amount of excess energy above the average energy of normal molecules are able to react to form products. This excess energy which a normal molecule must aquire in order to react is known as energy of activation (Ea). This energy of activation determines the rate of reaction. Higher the value of Ea, lower is the rate of reaction and greater stability. At higher
But in the case of enzyme catalyzed reaction, the rate of reaction is optimum at normal body temperature. Because all the molecules either energy-rich or energy-poor combine with the active site of the enzyme to form enzyme substrate complex. The latter breaks into enzyme and product. Thus, the enzyme acts by lowering the energy of activation of the reactions i.e. reducing the energy barrier and increases the rate of reaction.
Related Topics