Chapter: Biotechnology Applying the Genetic Revolution: Pathway Engineering
The Toluene/Xylene Pathway
THE
TOLUENE/XYLENE PATHWAY
The toluene/xylene pathway is
the best characterized of the aromatic degradation systems. It is carried on
the pTOL and pXYL plasmids. (Historically, these two types of plasmids were
isolated and named for the degradation of toluene or xylene. However, although
the plasmids differ in other respects they have essentially identical sets of
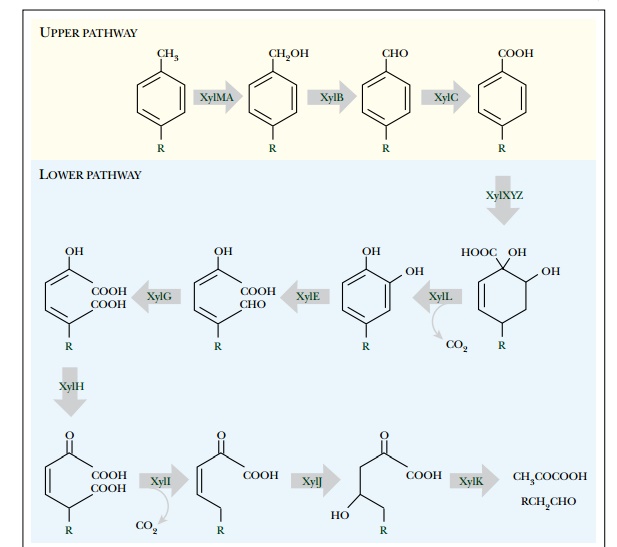
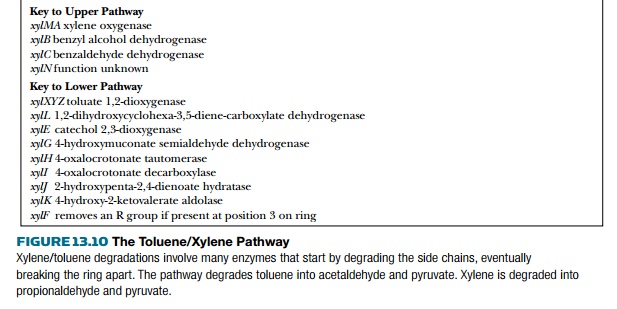
degradative genes.) The same pathway (Fig. 13.10) degrades toluene (R = H) and
xylene (R = CH3) as well as some derivatives. The “upper” pathway attacks the
side chains and the “lower” pathway breaks open the aromatic ring.
Ring dioxygenases such as
xylene or toluene oxygenase usually consist of three components.
The first component (e.g.,
the XylX protein of the xylene pathway) transfers reducing equivalents from NADH or NADPH via an enzyme
bound FAD to an Fe2S2 cluster. The second and third proteins (e.g., XylY and
XylZ) carry more Fe2S2 clusters and consume
molecular O2. Together they form the terminal oxygenase.
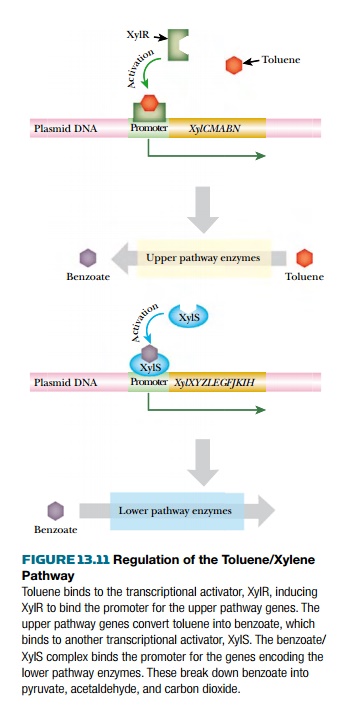
The xyl genes are induced by toluene or benzyl alcohol. These inducers
bind to the XylR protein, which activates the promoters for the upper pathway
genes (the xylCMABN operon) as well
as the xylS gene (Fig. 13.11). The
upper pathway produces benzoate, which acts as the inducer for the lower
pathway by binding to XylS protein. This then activates the lower pathway
promoter and induces the xylXYZLEGFJKIH
operon. (Promoters activated by the XylR protein require the alternative sigma
factor RpoN [= NtrA], which is normally needed by genes for nitrogen
metabolism. The significance of this is uncertain.)
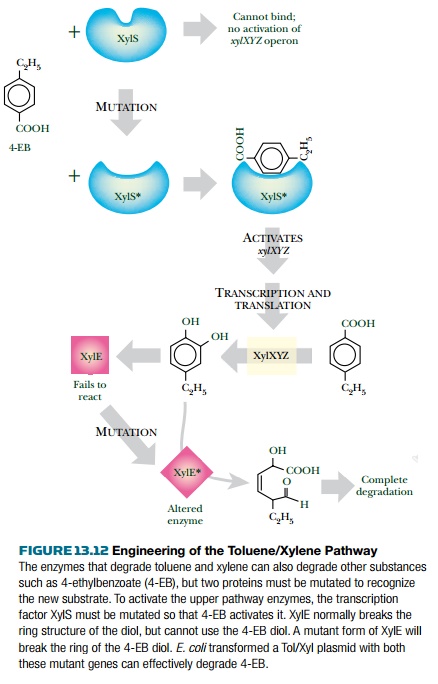
The Tol/Xyl system can be
engineered to accept substrates that are not originally used. For example, the
Xyl pathway cannot normally degrade the aromatic hydrocarbon, 4-ethylbenzoate
(4-EB). There are two problems. First, XylS activator protein does not
recognize 4-EB. However, xylS* mutants
have been selected that make an altered XylS protein, which has gained the
ability
to bind 4-EB and induces the
upper pathway. This converts 4-EB to 4-ethylcatechol. The second problem is
that the xylE product,
catechol-2,3-dioxygenase, is inhibited by 4-ethylcatechol. However, a xylE* mutant has been isolated that
makes an altered enzyme which not only is resistant to 4-ethylcatechol, but can
use it as a substrate. The xylS* xylE*
double mutant converts 4-ethylbenzoate completely to pyruvate plus acetaldehyde
(Fig. 13.12). Such an improved system can easily be transferred to other host
bacteria since it is already plasmid-borne.
Related Topics