Chapter: Biotechnology Applying the Genetic Revolution: Pathway Engineering
Indigo and Related Natural Pigments
INDIGO
AND RELATED NATURAL PIGMENTS
Enzymes that attack aromatic
rings often have a wide substrate range. Often they may work on related
compounds whose rings contain sulfur, oxygen, or nitrogen atoms, as well as on
hydrocarbons. The indole ring system is similar to naphthalene except that
indole contains a nitrogen in the ring.
Consequently, naphthalene
oxygenases also attack indole and its derivatives (Fig. 13.8).
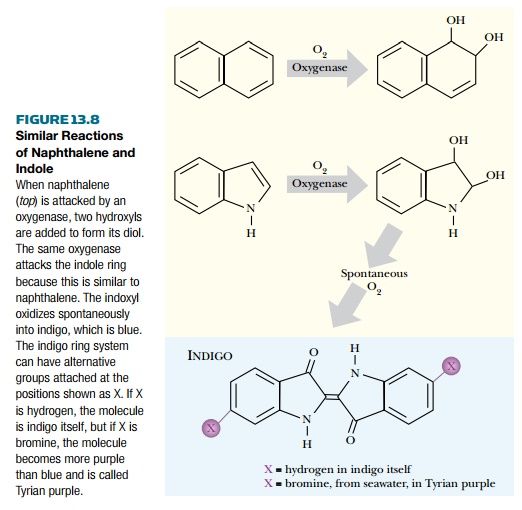
They convert indole to its
diol, which oxidizes spontaneously in air to yield indigo, a bright blue
pigment. In practice, most aromatic ring dioxygenases attack indole at least to
some extent. This allows rapid color screening for the presence of ring dioxygenases,
and the presumed aromatic pathways to which they a reddish purple dye, is made
by a sea snail called the spiny murex. A related snail, the banded murex,
secretes a mixture of Tyrian purple plus indigo, that is, presumably the lost
hyacinthine purple. Tyrian purple is very closely related to indigo (Fig.
13.8). It has two bromine atoms, extracted from seawater by the sea snail, on
the indigo ring. Both dyes are secreted as colorless precursors that turn blue
(or purple) by reacting with the oxygen in air. Indigo itself is used for
dyeing wool and cotton blue. Blue jeans are made of cotton dyed with indigo.
In earlier times, indigo was
extracted from plants, but nowadays it is chemically synthesized. Recently,
genetically altered bacteria that can make indigo were discovered, largely by
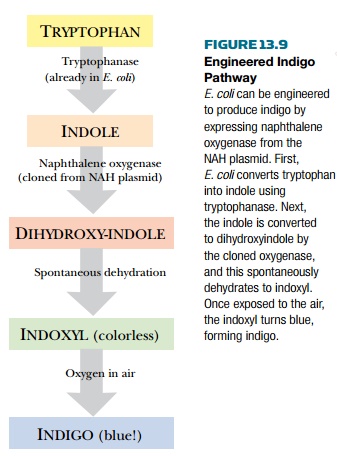
accident. The nah genes, carried on the NAH plasmid, encode the enzymes that
break down naphthalene. When the nah system was originally analyzed, genes from
the NAH plasmid were cloned into E. coli and some of the bacteria turned blue!
These blue bacteria turned out to possess the genes for naphthalene oxygenase,
the enzyme that carries out the first step in breaking down naphthalene. As
discussed earlier, naphthalene oxygenase works very well against indole and
converts it into indoxyl. Oxygen in the air converts indoxyl to indigo (see
Fig. 13.8). E. coli itself provides the indole by degrading the amino acid
tryptophan. Thus the engineered E. coli must be grown in rich medium containing
protein hydrolysate or some other source of tryptophan in order to generate
indigo (Fig. 13.9). Commercialization of such an engineered pathway would
involve putting the recombinant bacteria with the naphthalene oxygenase gene
onto a solid support in a bioreactor. Tryptophan would be added at one end and
indigo would emerge from the other.
Related Topics