Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Terminology Used In Coordination Chemistry
TERMINOLOGY USED IN COORDINATION CHEMISTRY
(a) Lewis Acid
All electron acceptors are lewis acids.
(b)
Lewis Base
All electron donors are lewis base.
(c) Central metal ion
In the complex ion an acceptor accepts a pair of
electrons from the donor atoms. The
acceptor is usually a metal / metal ion to which one (or) more of neutral molecules (or) anions are attached. The acceptor
metal cation is referred to as central metal
cation. Hence, central metal cation in a complex serves as a lewis acid.
(d) Oxidation state
This number denotes the charge, explaining the number of
electrons it has lost to form the
cation. It is oxidation number that denotes the charge, if the central metal atom would have if all the ligand in the
complex were removed along with their
electron pairs that were shared with the central atom. It is usually represented by Roman Numeral.
(e) Ligand (Latin word meaning to bind)
A ligand is an ion (or) a molecule capable of
functioning as an electron donor.
Therefore the neutral molecules or ions which are directly attached to the central metal ion are called as ligand (or) coordination
groups. These coordination groups or
ligands can donate a pair of electrons to the central metal ion (or) atom. Hence, in a complex compound ligands act as
Lewisbases.
Types of ligands
When a ligand is bound to a metal ion through a single
donor atom, as with - Cl , H2O or NH3, the ligand is said to be unidentate.
Whenever a single coordinating group (or)
ligand occupies two (or) more coordination position on
the same central metal ions, a complex possessing a
closed ring is formed. Such ligands are
called polydentate ligands. When a single ligand has two coordinating positions,itiscalledbidentateligandandwhentherearethreecoordinatingpositions
available, it is called a tridentate ligand and so on.
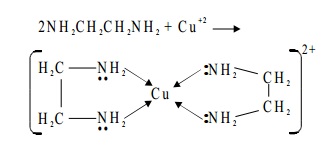
For example, ethylenediamine is a
bidentate ligand because it has two amino groups each of which can donate a pair of electrons.
Name of the ligands
Positive ligands
The positive ligands are named with an ending -ium.
NH2 - NH3+ hydrazinium
This ligand, though positive can bind through the
uncharged nitrogen.
Neutral ligands
The neutral ligands are named as such without any
special name. But water is written as
'aqua : Ammonia is written as ammine. Note that two m's to distinguish from organic amine CO-Carbonyl,
NO-Nitrosyl, NH2 - CH2 - CH2 - NH2-ethylenediamine (en), Pyridine C5H5N.
Negative Ligands
Negative ligands end in suffix 'O'.
Example
F--Fluoro, Cl--Chloro, C2O42--Oxalato,
CN--Cyano,
NO2--Nitro,
Br--Bromo, SO42--Sulphato, CH3COO--acetato CNS--thiocyanato, NCS--isothiocyanato, S2O32--thiosulphato.
Chelates
If a ligand is capable of forming more than one bond with
the central metal atom (or) ion then
the ring structures are produced which are known as metal chelates. Hence the
ring forming group are described as chelating agents (or) polydentate ligands.
Coordination sphere
In a complex compound, it usually, central metal ion and
the ligands are enclosed with in
square bracket is called as coordination sphere. This represents a single constituent unit. The ionisable species are
placed outside the square bracket.
These ions do not ionise to give the test for
constituent ions.
Coordination number
The coordination number of a metal ion in a complex can
be defined as the number of ligand
donor atoms to which the metal is directly bonded. Numerically coordination number represents the total number of the
chemical bonds formed between the
central metal ion and the donor atoms of the ligands. For example in K4[Fe(CN)6] the coordination number of Fe(II) is 6 and in [Cu(NH3)4]SO4 the coordination
number of Cu(II) is 4.
Charge on the complex ion
Charge on the complex ion is equal to the sum of the
charges on the metal
ion and their ligands.
Example
1. [Cu(NH3)4]2+ can be
written as [Cu2+(NH3)4]2+ since NH3 ligand is neutral. The sum of the charges on the metal ion and the ligands
= +2.
This can be determined as shown below
Charge on the metal ion (Cu2+) = +2
Charge on the ligand (NH3) = 4 × 0 = 0
Net charge on the complex ion = +2 + 0 = +2
2. Similarly for
[Fe(CN)6]4- (or) [Fe2+(CN)6]4-
The sum of the charge on the metal ion and the ligand =
-4.
Charge on the metal ion (Fe2+) = +2
Charge on the ligand (CN-) = 6 × (-1) = -6
Net
charge on the complex = +2 - 6 = -4
Related Topics