Chapter: Basic & Clinical Pharmacology : Cancer Chemotherapy
Taxanes & Related Drugs
TAXANES & RELATED DRUGS
Paclitaxel is an alkaloid ester derived from the Pacific yew (Taxusbrevifolia) and the European yew (Taxus baccata). The drug func-tions as a
mitotic spindle poison through high-affinity binding to microtubules with enhancement of tubulin polymerization.
This promotion of microtubule assembly by paclitaxel occurs in the absence of
microtubule-associated proteins and guanosine triphos-phate and results in
inhibition of mitosis and cell division.
Paclitaxel has
significant activity in a broad range of solid tumors, including ovarian,
advanced breast, non-small cell and small cell lung, head and neck, esophageal,
prostate, and bladder cancers and AIDS-related Kaposi’s sarcoma. It is
metabolized extensively by the liver P450 system, and nearly 80% of the drug is
excreted in feces via the hepatobiliary route. Dose reduction is required in
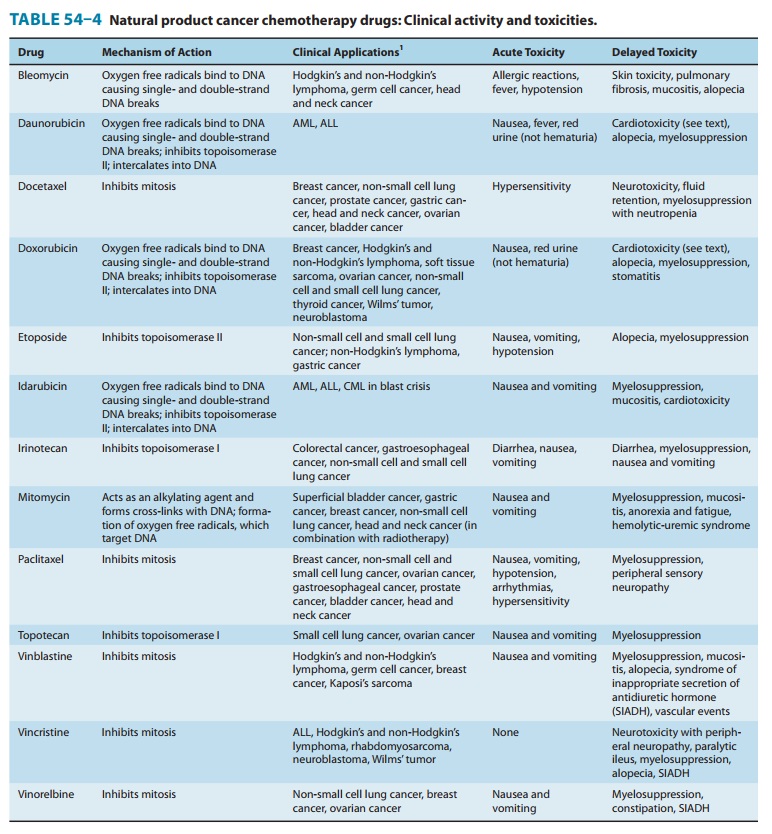
patients with liver dysfunction. The primary dose-limiting toxicities are
listed in Table 54–4. Hypersensitivity reac-tions may be observed in up to 5%
of patients, but the incidence is significantly reduced by premedication with
dexamethasone, diphenhydramine, and an H2 blocker.
A
novel albumin-bound paclitaxel formulation (Abraxane) is approved for use in
metastatic breast cancer. In contrast to paclitaxel, this formulation is not
associated with hypersensitivity reactions, and premedication to prevent such
reactions is not required. Moreover, this agent has significantly reduced
myelosuppressive effects com-pared with paclitaxel, and the neurotoxicity that
results appears to be more readily reversible than is typically observed with
paclitaxel.
Docetaxel is
a semisynthetic taxane derived from the Europeanyew tree. Its mechanism of
action, metabolism, and elimination are identical to those of paclitaxel. It is
approved for use as second-line therapy in advanced breast cancer and non-small
cell lung cancer, and it also has major activity in head and neck cancer, small
cell lung cancer, gastric cancer, advanced platinum-refrac-tory ovarian cancer,
and bladder cancer. Its major toxicities are listed in Table 54–4.
Cabazitaxel is
a semisynthetic taxane produced from a precursorextracted from the yew tree.
Its mechanism of action, metabolism, and elimination are identical to those of
the other taxanes. However, unlike other taxanes, cabazitaxel is a poor
substrate for the multi-drug resistance P-glycoprotein efflux pump and may
therefore be useful for treating multidrug-resistant tumors. It is approved for
use in combination with prednisone in the second-line therapy of
hor-mone-refractory metastatic prostate cancer previously treated with a
docetaxel-containing regimen. Its major toxicities include myelo-suppression,
neurotoxicity, and allergic reactions.
Although
not strictly a taxane, ixabepilone
is a semisynthetic epothilone B analog that functions as a microtubule
inhibitor and binds directly to β-tubulin subunits on microtubules,
leading to inhibition of normal microtubule dynamics. As such, it is active in
the M phase of the cell cycle. This agent is presently approved for metastatic
breast cancer in combination with the oral fluoropy-rimidine capecitabine or as
monotherapy. Of note, this agent continues to have activity in drug-resistant
tumors that overex-press P-glycoprotein or tubulin mutations. The main adverse
effects include myelosuppression, hypersensitivity reactions, and neurotoxicity
in the form of peripheral sensory neuropathy.
Related Topics