Chapter: Basic & Clinical Pharmacology : Cancer Chemotherapy
Alkylating Agents - Pharmacology of Cancer Chemotherapeutic Drugs
BASIC PHARMACOLOGY OF CANCER CHEMOTHERAPEUTIC DRUGS
ALKYLATING AGENTS
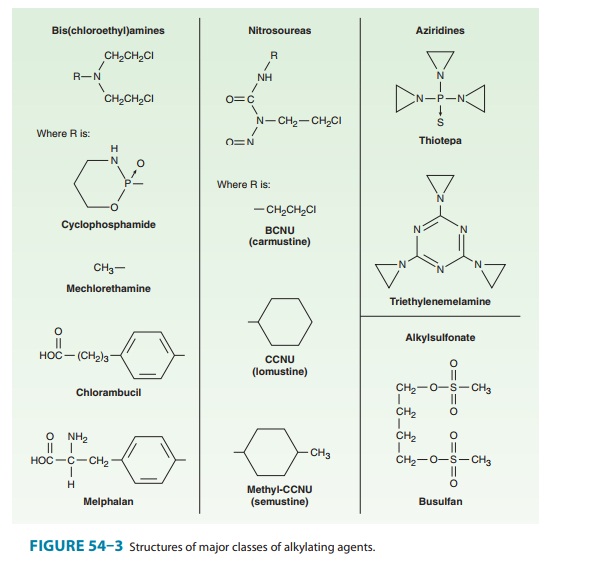
The major clinically
useful alkylating agents (Figure 54–3) have a structure containing a
bis(chloroethyl)amine, ethyleneimine, or nitrosourea moiety, and they are
classified in several different groups. Among the bis(chloroethyl)amines,
cyclophosphamide, mechlorethamine, melphalan, and chlorambucil are the most
use-ful. Ifosfamide is closely related to cyclophosphamide but has a somewhat
different spectrum of activity and toxicity. Thiotepa and busulfan are used to
treat breast and ovarian cancer, and chronic myeloid leukemia, respectively.
The major nitrosoureas are carmustine (BCNU) and lomustine (CCNU).
Mechanism of Action
As a class, the
alkylating agents exert their cytotoxic effects via transfer of their alkyl
groups to various cellular constituents. Alkylations of DNA within the nucleus
probably represent the major interactions that lead to cell death. However,
these drugs react chemically with sulfhydryl, amino, hydroxyl, carboxyl, and
phosphate groups of other cellular nucleophiles as well. The general mechanism
of action of these drugs involves intramolecular cycliza-tion to form an
ethyleneimonium ion that may directly or through formation of a carbonium ion
transfer an alkyl group to a cellular constituent (Figure 54–4). In addition to
alkylation, a secondary mechanism that occurs with nitrosoureas involves
carbamoylation of lysine residues of proteins through formation of isocyanates.
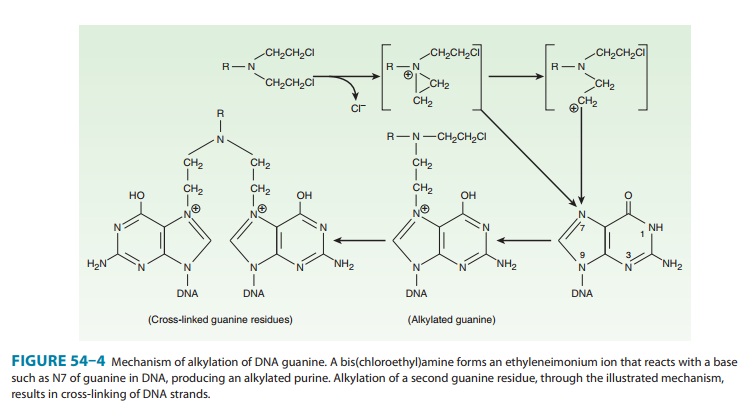
The major site of
alkylation within DNA is the N7 position of guanine; however, other bases are
also alkylated albeit to lesser degrees, including N1 and N3 of adenine, N3 of
cytosine, and O6 of guanine, as well as phosphate atoms and proteins associated
with DNA. These interactions can occur on a single strand or on
Alkylation of guanine can result in
miscoding through abnormal base pairing with thymine or in depurination by
excision of guanine residues. The latter effect leads to DNA strand breakage
through scission of the sugar-phosphate backbone of DNA. Cross-linking of DNA
appears to be of major importance to the cytotoxic action of alky-lating
agents, and replicating cells are most susceptible to these drugs. Thus,
although alkylating agents are not cell cycle specific, cells are most
susceptible to alkylation in late G1
and S phases of the cell cycle.
Resistance
The mechanism of
acquired resistance to alkylating agents may involve increased capability to
repair DNA lesions, decreased trans-port of the alkylating drug into the cell,
and increased expression or activity of glutathione and glutathione-associated
proteins, which are needed to conjugate the alkylating agent, or increased
glutathione S-transferase activity,
which catalyzes the conjugation.
Adverse Effects
The
adverse effects usually associated with alkylating agents are generally
dose-related and occur primarily in rapidly growing tis-sues such as bone
marrow, gastrointestinal tract, and reproductive system. Nausea and vomiting
can be a serious issue with a number of these agents. In addition, they are
potent vesicants and can damage tissues at the site of administration as well
as producesystemic toxicity. As a class, alkylating agents are carcinogenic in
nature, and there is an increased risk of secondary malignancies, especially
acute myelogenous leukemia.
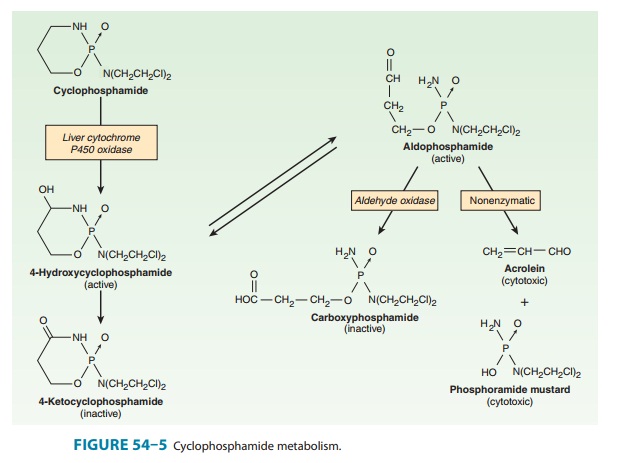
Cyclophosphamide is
one of the most widely used alkylating agents. One of the potential advantages
of this compound relates to its high oral bioavailability. As a result, it can be
administered via the oral and intravenous routes with equal clinical efficacy.
It is inactive in its parent form, and must be activated to cytotoxic forms by
liver microsomal enzymes (Figure 54–5). The cyto-chrome P450 mixed-function
oxidase system converts cyclophos-phamide to 4-hydroxycyclophosphamide, which
is in equilibrium with aldophosphamide. These active metabolites are delivered
to both tumor and normal tissue, where nonenzymatic cleavage of aldophosphamide
to the cytotoxic forms—phosphoramide mus-tard and acrolein—occurs. The liver
appears to be protected through the enzymatic formation of the inactive
metabolites 4-ketocyclophosphamide and carboxyphosphamide.
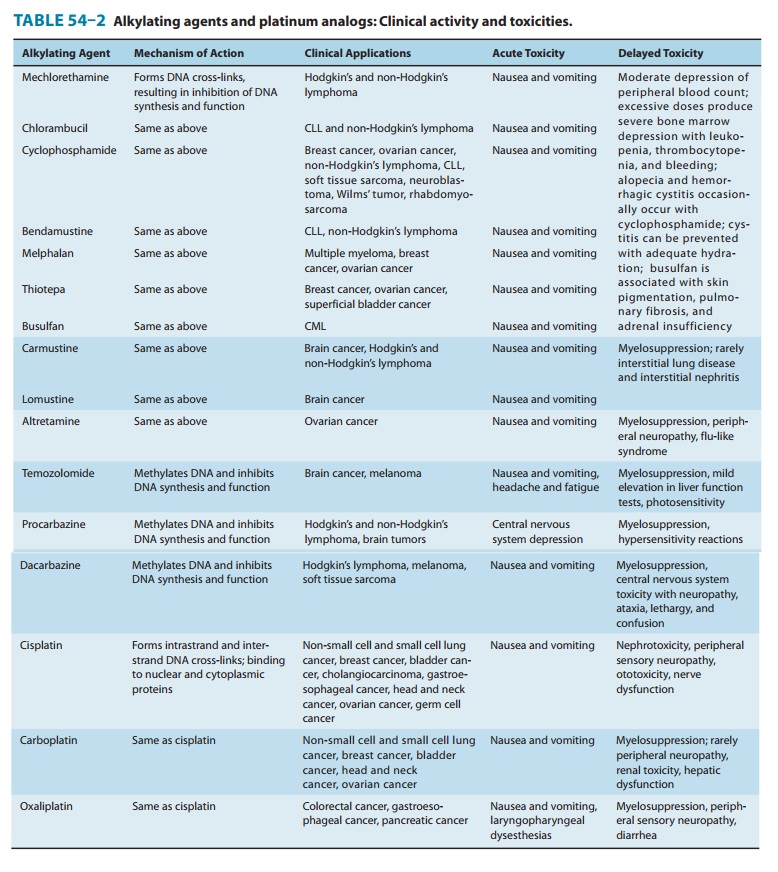
The major toxicities
of the individual alkylating agents are outlined in Table 54–2 and discussed
below.
Related Topics