Chapter: Basic & Clinical Pharmacology : Cancer Chemotherapy
Role of Cell Cycle Kinetics & Anticancer Effect
ROLE OF CELL CYCLE KINETICS &
ANTICANCER EFFECT
The key principles of
cell cycle kinetics were initially developed using the murine L1210 leukemia as
the experimental model system (Figure 54–1). However, drug treatment of human
cancers requires a clear understanding of the differences between the
char-acteristics of this rodent leukemia and of human cancers, as well as an
understanding of the differences in growth rates of normal target tissues
between mice and humans. For example, L1210 is a rapidly growing leukemia with
a high percentage of cells synthesiz-ing DNA, as measured by the uptake of
tritiated thymidine (the labeling index). Because L1210 leukemia has a growth
fraction of 100% (ie, all its cells are actively progressing through the cell
cycle), its life cycle is consistent and predictable. Based on the murine L1210
model, the cytotoxic effects of anticancer drugs follow log cell-kill kinetics.
As such, a given agent would be predicted to kill a constant fraction of cells
as opposed to a constant number.
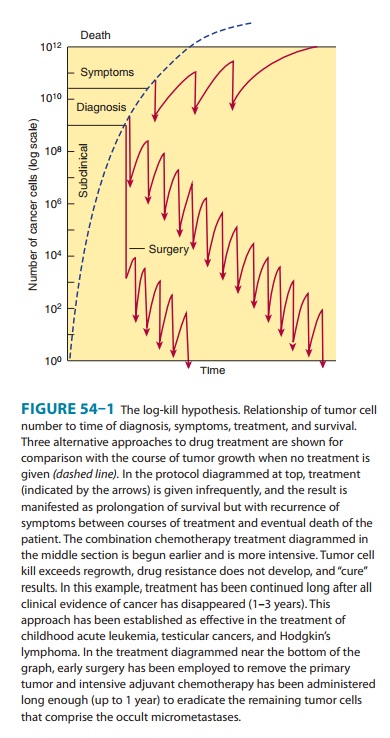
Thus, if a particular
dose of an individual drug leads to a 3 log kill of cancer cells and reduces
the tumor burden from 1010 to 107 cells, the same dose used at a tumor burden of 105 cells reduces the
tumor mass to 102 cells. Cell kill is, therefore, proportional, regardless of
tumor burden. The cardinal rule of chemotherapy— the invariable inverse
relation between cell number and curability—was established with this model,
and this relationship is applicable to other hematologic malignancies.
Although growth of murine leukemias simulates exponential cell kinetics, mathematical modeling data suggest that most human solid tumors do not grow in such an exponential manner. Taken together, the experimental data in human solid cancers support a Gompertzian model of tumor growth and regression. The critical distinction between Gompertzian and exponential growth is that the growth fraction of the tumor is not constant with Gompertzian kinetics but instead decreases exponentially with time (exponential growth is matched by exponential retarda-tion of growth, due to blood supply limitations and other fac-tors). The growth fraction peaks when the tumor is approximately one third its maximum size. Under the Gompertzian model, when a patient with advanced cancer is treated, the tumor mass is larger, its growth fraction is low, and the fraction of cells killed is, there-fore, small. An important feature of Gompertzian growth is that response to chemotherapy in drug-sensitive tumors depends, in large measure, on where the tumor is in its particular growth curve.
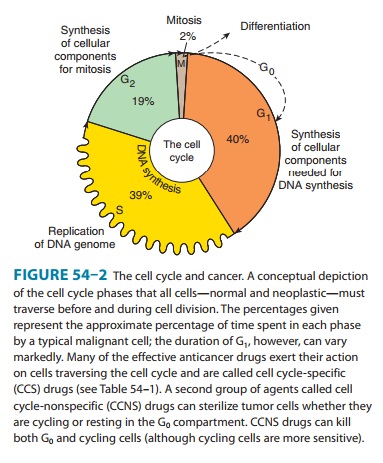
Information on cell
and population kinetics of cancer cells explains, in part, the limited
effectiveness of most available anti-cancer drugs. A schematic summary of cell
cycle kinetics is pre-sented in Figure 54–2. This information is relevant to
the mode of action, indications, and scheduling of cell cycle-specific (CCS)
and cell cycle-nonspecific (CCNS) drugs. Agents falling into these two major
classes are summarized in Table 54–1.
The Role of Drug Combinations
With rare exceptions (eg, choriocarcinoma and Burkitt’s lym-phoma), single drugs at clinically tolerable doses have been unable to cure cancer. In the 1960s and early 1970s, drug combination regimens were developed based on the known biochemical actions of available anticancer drugs rather than on their clinical efficacy.
Such regimens were,
however, largely ineffective. The era of effec-tive combination chemotherapy
began when a number of active drugs from different classes became available for
use in combina-tion in the treatment of the acute leukemias and lymphomas.
Following this initial success with hematologic malignancies, com-bination
chemotherapy was extended to the treatment of solid tumors.
The
use of combination chemotherapy is important for several reasons. First, it
provides maximal cell kill within the range of toxicity tolerated by the host
for each drug as long as dosing is not compromised. Second, it provides a broader
range of interaction between drugs and tumor cells with different genetic
abnormali-ties in a heterogeneous tumor population. Finally, it may prevent or
slow the subsequent development of cellular drug resistance. The same
principles apply to the therapy of chronic infections, such as HIV and
tuberculosis.
Certain
principles have guided the selection of drugs in the most effective drug
combinations, and they provide a paradigm for the development of new drug
therapeutic programs.
1. Efficacy: Only drugs known to be somewhat effective whenused alone against
a given tumor should be selected for use in combination. If available, drugs
that produce complete remis-sion in some fraction of patients are preferred to
those that produce only partial responses.
2. Toxicity: When several drugs of a given class are available andare equally effective, a drug should be selected on the basis of toxicity that does not overlap with the toxicity of other drugs in the combination. Although such selection leads to a wider range of adverse effects, it minimizes the risk of a lethal effect caused by multiple insults to the same organ system by differ-ent drugs and allows dose intensity to be maximized.
3.
Optimum scheduling: Drugs should be used
in their optimaldose and schedule, and drug combinations should be given at
consistent intervals. Because long intervals between cycles negatively affect
dose intensity, the treatment-free interval between cycles should be the
shortest time necessary for recov-ery of the most sensitive normal target
tissue, which is usually the bone marrow.
4. Mechanism
of interaction: There should be a clear under-standing of the
biochemical, molecular, and pharmacokinetic mechanisms of interaction between
the individual drugs in a given combination, to allow for maximal effect.
Omission of a drug from a combination may allow overgrowth by a tumor clone
sensitive to that drug alone and resistant to other drugs in the combination.
5.
Avoidance of arbitrary
dose changes: An
arbitrary reductionin the dose of an effective drug in order to add other less
effec-tive drugs may reduce the dose of the most effective agent below the
threshold of effectiveness and destroy the ability of the combination to cure
disease in a given patient.
Dosage Factors
Dose intensity is one
of the main factors limiting the ability of chemotherapy or radiation therapy
to achieve cure. The dose-response curve in biologic systems is usually
sigmoidal in shape, with a threshold, a linear phase, and a plateau phase. For
chemo-therapy, therapeutic selectivity is dependent on the difference between
the dose-response curves of normal and tumor tissues. In experimental animal
models, the dose-response curve is usually steep in the linear phase, and a
reduction in dose when the tumor is in the linear phase of the dose-response
curve almost always results in a loss in the capacity to cure the tumor
effectively before a reduction in the antitumor activity is observed. Although
com-plete remissions continue to be observed with dose reduction as low as 20%,
residual tumor cells may not be entirely eliminated, thereby allowing for
eventual relapse. Because anticancer drugs are associated with toxicity, it is
often appealing for clinicians to avoid acute toxicity by simply reducing the
dose or by increasing the time interval between each cycle of treatment.
However, such empiric modifications in dose represent a major cause of
treatment failure in patients with drug-sensitive tumors.
A
positive relationship between dose intensity and clinical effi-cacy has been
documented in several solid tumors, including advanced ovarian, breast, lung,
and colon cancers, as well as in hematologic malignancies, such as the
lymphomas. At present, there are three main approaches to dose-intense delivery
of chemotherapy. The first approach, dose
escalation, involves increasing the doses of the respective anticancer
agents. The second strategy is administra-tion of anticancer agents in a
dose-intense manner by reducing
theinterval between treatment cycles, while the third approach involves sequential scheduling of either single
agents or of combinationregimens. Each of these strategies is presently being
applied to a wide range of solid cancers, including breast, colorectal, and non-small
cell lung, and in general, such dose-intense regimens have significantly
improved clinical outcomes.
Related Topics