Chapter: Basic & Clinical Pharmacology : Cancer Chemotherapy
Purine Antagonists
PURINE ANTAGONISTS
6-Thiopurines
6-Mercaptopurine
(6-MP) was the first of the thiopurine analogs found to be effective in cancer
therapy. This agent is used primarily in the treatment of childhood acute
leukemia, and a closely related analog, azathioprine, is used as an
immunosuppressive agent . As with other thiopurines, 6-MP is inactive in its
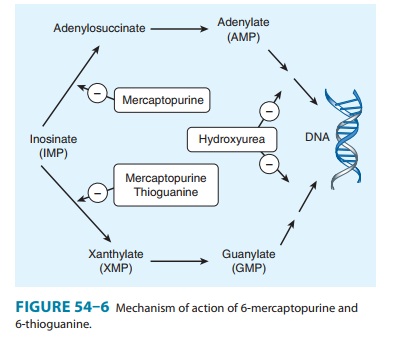
parent form and must be metabolized by hypoxanthine-guanine phosphoribosyl
transferase (HGPRT) to form the monophosphate nucleotide 6-thioinosinic acid,
which in turn inhibits several enzymes of de novo purine nucleotide synthesis
(Figure 54–6). The monophosphate form is eventually metabolized to the
triphosphate form, which can then be incorporated into both RNA and DNA.
Significant levels of thioguanylic acid and 6-methylmercaptopurine ribotide
(MMPR) are also formed from 6-MP. These metabolites may contribute to its
cytotoxic action.
6-Thioguanine
(6-TG) also inhibits several enzymes in the de novo purine nucleotide
biosynthetic pathway (Figure 54–6). Various metabolic lesions result, including
inhibition of purine nucleotide interconversion; decrease in intracellular
levels of gua-nine nucleotides, which leads to inhibition of glycoprotein
synthe-sis; interference with the formation of DNA and RNA; and incorporation
of thiopurine nucleotides into both DNA and RNA. 6-TG has a synergistic action
when used together with cytarabine in the treatment of adult acute leukemia.
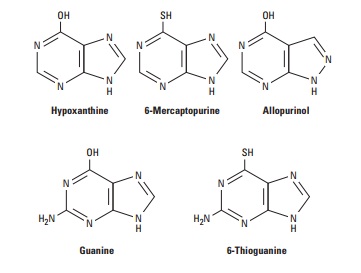
6-MP is converted to an inactive metabolite (6-thiouric acid) by an oxidation reaction catalyzed by xanthine oxidase, whereas 6-TG undergoes deamination. This is an important issue because the purine analog allopurinol, a potent xanthine oxidase inhibitor, is frequently used as a supportive care measure in the treatment of acute leukemias to prevent the development of hyperuricemia that often occurs with tumor cell lysis. Because allopurinol inhibits xan-thine oxidase, simultaneous therapy with allopurinol and 6-MP would result in increased levels of 6-MP, thereby leading to excessive toxicity. In this setting, the dose of mercaptopurine must be reduced by 50–75%. In contrast, such an interaction does not occur with 6-TG, which can be used in full doses with allopurinol.
The
thiopurines are also metabolized by the enzyme thiopurine methyltransferase
(TPMT), in which a methyl group is attached to the thiopurine ring. Patients
who have a pharmacogenetic syndrome involving partial or complete deficiency of
this enzyme are at increased risk for developing severe toxicities in the form
of myelosuppression and gastrointestinal toxicity with mucositis and diarrhea.
Fludarabine
Fludarabine
phosphate is rapidly dephosphorylated to 2-fluoro-arab-inofuranosyladenosine
and then phosphorylated intracellularly by deoxycytidine kinase to the
triphosphate. The triphosphate metabo-lite interferes with the processes of DNA
synthesis and DNA repair through inhibition of DNA polymerase-α and DNA
polymerase-β.
The triphosphate form can also be directly incorporated into DNA, resulting in
inhibition of DNA synthesis and function. The diphos-phate metabolite of
fludarabine inhibits ribonucleotide reductase, leading to inhibition of
essential deoxyribonucleotide triphosphates. Finally, fludarabine induces
apoptosis in susceptible cells through as yet undetermined mechanisms. This
purine nucleotide analog is used mainly in the treatment of low-grade
non-Hodgkin’s lymphoma and chronic lymphocytic leukemia (CLL). It is given
parenterally, and up to 25–30% of parent drug is excreted in the urine. The
main dose-limiting toxicity is myelosuppression. This agent is a potent
immuno-suppressant with inhibitory effects on CD4 and CD8 T cells. Patients are
at increased risk for opportunistic infections, including fungi, herpes, and Pneumocystis jiroveci pneumonia (PCP).
Patients should receive PCP prophylaxis with trimethoprim-sulfamethoxazole (dou-ble
strength) at least three times a week, and this should continue for up to 1
year after stopping fludarabine therapy.
Cladribine
Cladribine
(2-chlorodeoxyadenosine) is a purine nucleoside analog with high specificity
for lymphoid cells. Inactive in its parent form, it is initially phosphorylated
by deoxycytidine kinase to the mono-phosphate form and eventually metabolized
to the triphosphateform, which can then be incorporated into DNA. The
triphos-phate metabolite can also interfere with DNA synthesis and DNA repair
by inhibiting DNA polymerase-α and DNA polymerase-β, respectively. Cladribine is indicated for
the treatment of hairy cell leukemia, with activity in other low-grade lymphoid
malignancies such as CLL and low-grade non-Hodgkin’s lymphoma. It is nor-mally
administered as a single continuous 7-day infusion; under these conditions, it
has a very manageable safety profile with the main toxicity consisting of
transient myelosuppression. As with other purine nucleoside analogs, it has
immunosuppressive effects, and a decrease in CD4 and CD8 T cells, lasting for
over 1 year, is observed in patients.
Related Topics