Chapter: Basic & Clinical Pharmacology : Cancer Chemotherapy
Camptothecins
CAMPTOTHECINS
The camptothecins are
natural products derived from the Camptotheca
acuminata tree originally found in China; theyinhibit the activity of
topoisomerase I, the key enzyme responsible for cutting and religating single
DNA strands. Inhibition of this enzyme results in DNA damage. Topotecan and irinotecan are the two camptothecin analogs used in clinical
practice in the USA. Although they both inhibit the same molecular target,
their spec-trum of clinical activity is quite different. Topotecan is indicated
in the treatment of advanced ovarian cancer as second-line therapy following
initial treatment with platinum-based chemotherapy. It is also approved as
second-line therapy of small cell lung cancer. The main route of elimination is
renal excretion, and dosage must be adjusted in patients with renal impairment.
Irinotecan
is a prodrug that is converted mainly in the liver by the carboxylesterase
enzyme to the SN-38 metabolite, which is 1000-fold more potent as an inhibitor
of topoisomerase I than the parent compound. In contrast to topotecan,
irinotecan and SN-38 are mainly eliminated in bile and feces, and dose
reduction is required in the setting of liver dysfunction. Irinotecan was originally
approved as second-line monotherapy in patients with metastatic colorectal
cancer who had failed fluorouracil-based therapy. It is now approved as
first-line therapy when used in combination with 5-FU and leucovorin.
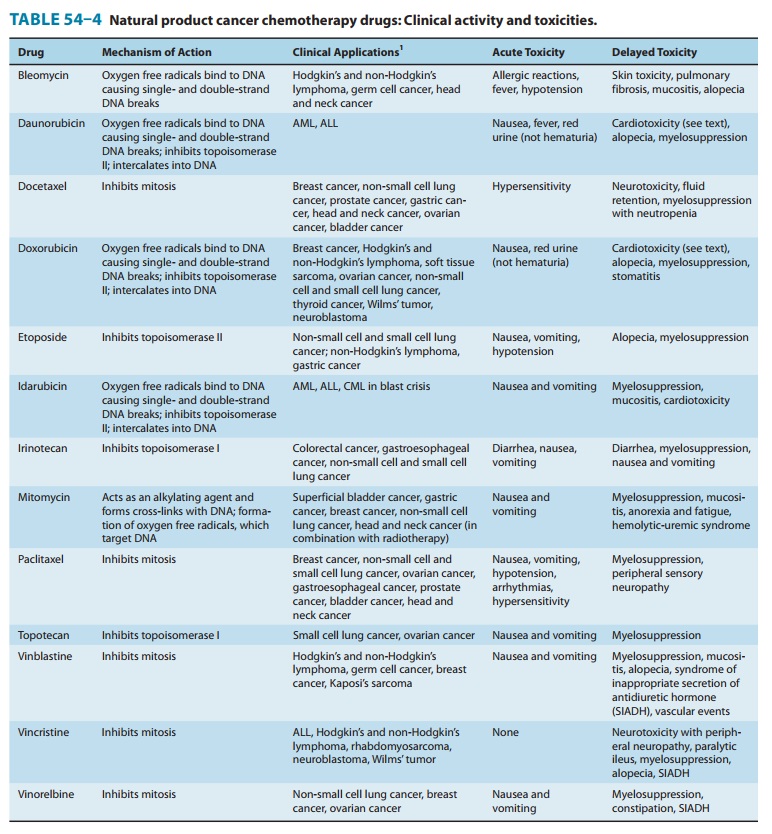
Myelosuppression and diarrhea are the two most common adverse events (Table
54–4). There are two forms of diarrhea: an early form that occurs within 24
hours after administra-tion and is thought to be a cholinergic event
effectively treated with atropine, and a late form that usually occurs 2–10
days after treat-ment. The late diarrhea can be severe, leading to significant
electro-lyte imbalance and dehydration in some cases.
Related Topics