Chapter: Basic & Clinical Pharmacology : Cancer Chemotherapy
Platinum Analogs
PLATINUM ANALOGS
Three
platinum analogs are currently used in clinical practice: cis-platin,
carboplatin, and oxaliplatin. Cisplatin (cis-diamminedichlo-roplatinum [II]) is
an inorganic metal complex that was initially discovered through a
serendipitous observation that neutral plati-num complexes inhibited division
and filamentous growth of Escherichia
coli. Several platinum analogs were subsequently synthe-sized. Although the
precise mechanism of action of the platinum analogs is unclear, they are
thought to exert their cytotoxic effects in the same manner as alkylating
agents. As such, they kill tumor cells in all stages of the cell cycle and bind
DNA through the formation of intrastrand and interstrand cross-links, thereby
leading to inhibi-tion of DNA synthesis and function. The primary binding site
is the N7 position of guanine, but covalent interaction with the N3 posi-tion
of adenine and O6 position of cytosine can also occur. In addi-tion to
targeting DNA, the platinum analogs have been shown to bind to both cytoplasmic
and nuclear proteins, which may also contribute to their cytotoxic and
antitumor effects. The platinum complexes appear to synergize with certain
other anticancer drugs, including alkylating agents, fluoropyrimidines, and
taxanes.

Cisplatin has major antitumor
activity in a broad range of solid tumors, including non-small cell and small
cell lung cancer, esophageal and gastric cancer, cholangiocarcinoma, head and
neck cancer, and genitourinary cancers, particularly testicular, ovarian, and
bladder cancer. When used in combination regimens, cispla-tin-based therapy has
led to the cure of nonseminomatous testicu-lar cancer. Cisplatin and the other
platinum analogs are extensively cleared by the kidneys and excreted in the
urine. As a result, dose modification is required in patients with renal
dysfunction.
Carboplatin is a
second-generation platinum analog whose mechanisms of cytotoxic action,
mechanisms of resistance, and clinical pharmacology are identical to those
described for cisplatin.
As
with cisplatin, carboplatin has broad-spectrum activity against a wide range of
solid tumors. However, in contrast to cisplatin, it exhibits significantly less
renal toxicity and gastrointestinal toxicity. Its main dose-limiting toxicity
is myelosuppression. It has therefore been widely used in transplant regimens
to treat refractory hema-tologic malignancies. Moreover, since vigorous
intravenous hydra-tion is not required for carboplatin therapy, carboplatin is
viewed as an easier agent to administer to patients, and as such, it has
replaced cisplatin in various combination chemotherapy regimens.
Oxaliplatin is a
third-generation diaminocyclohexane plati-num analog. Its mechanism of action
and clinical pharmacology are identical to those of cisplatin and carboplatin.
However, tumors that are resistant to cisplatin or carboplatin on the basis of
mismatch repair defects are not cross-resistant to oxaliplatin, and this
finding may explain the activity of this platinum compound in colorectal
cancer. Oxaliplatin was initially approved for use as second-line therapy in
combination with the fluoropyrimidine 5-fluorouracil (5-FU) and leucovorin,
termed the FOLFOX regi-men, for metastatic colorectal cancer. The FOLFOX
regimen was subsequently (2005) approved for the first-line treatment of
meta-static colorectal cancer. At this time, oxaliplatin-based chemo-therapy
has also been approved in the adjuvant therapy of high-risk stage II and stage
III colon cancer. Clinical activity has been observed in other gastrointestinal
cancers, such as pancreatic, gastroesophageal, and hepatocellular cancer. In
the side effect profile, neurotoxicity is the main dose-limiting toxicity, and
is manifested by a peripheral sensory neuropathy. There are two forms of
neurotoxicity, an acute form that is often triggered and worsened by exposure
to cold, and a chronic form that is dose-dependent. Although this chronic form
is dependent on the cumulative dose of drug administered, it tends to be
reversible, in contrast to cisplatin-induced neurotoxicity.
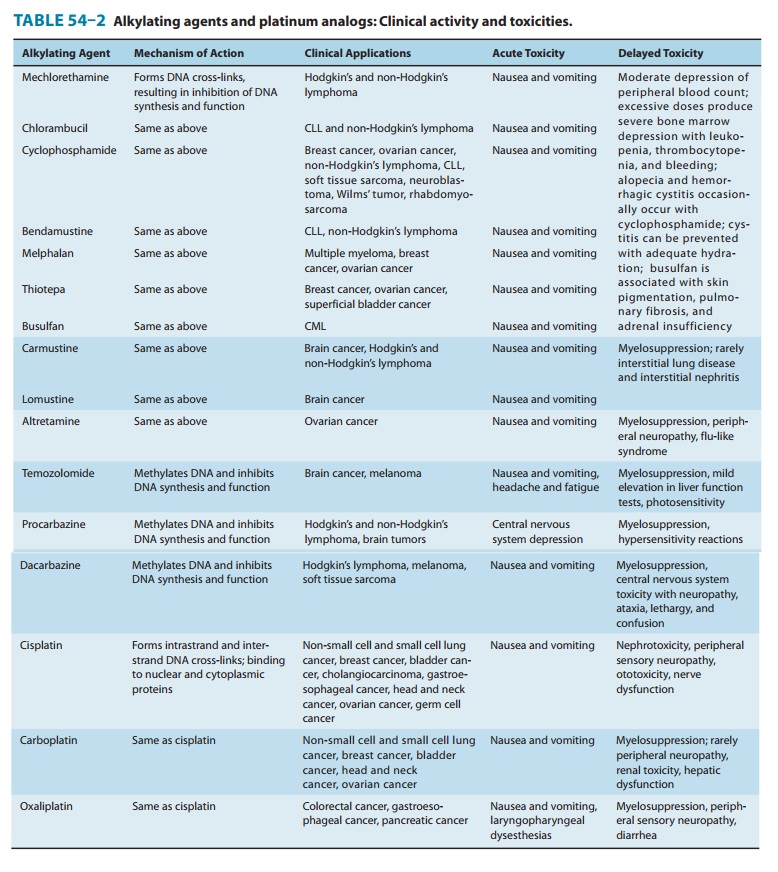
The major toxicities
of the individual platinum analogs are outlined in Table 54–2.
Related Topics