Chapter: Basic & Clinical Pharmacology : Cancer Chemotherapy
Antitumor Antibiotics
ANTITUMOR ANTIBIOTICS
Screening of microbial products has led to the discovery of a number of growth-inhibiting compounds that have proved to be clini-cally useful in cancer chemotherapy. Many of these antibiotics bind to DNA through intercalation between specific bases and block the synthesis of RNA, DNA, or both; cause DNA strand scission; and interfere with cell replication. All of the anticancer antibiotics now being used in clinical practice are products of various strains of the soil microbeStreptomyces. These include the anthracyclines, bleomycin, and mitomycin.
ANTHRACYCLINES
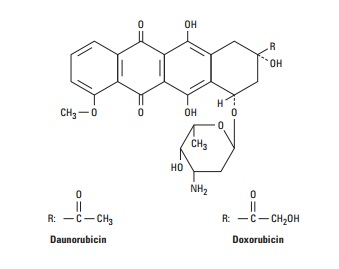
The anthracycline antibiotics, isolated from Streptomyces peucetius var caesius, are among the most widely used cytotoxic anticancer drugs. The structures of two congeners, doxorubicin and daunoru-bicin, are shown below. Several other anthracycline analogs have entered clinical practice, including idarubicin, epirubicin, and mitoxantrone. The anthracyclines exert their cytotoxic action through four major mechanisms: (1) inhibition of topoisomerase II; (2) high-affinity binding to DNA through intercalation, with consequent blockade of the synthesis of DNA and RNA, and DNA strand scission; (3) generation of semiquinone free radicals and oxygen free radicals through an iron-dependent, enzyme-mediated reductive process; and (4) binding to cellular membranes to alter fluidity and ion transport. While the precise mechanisms by which the anthracyclines exert their cytotoxic effects remain to be defined (and may depend upon the specific tumor type), it is now well-es-tablished that the free radical mechanism is the cause of the cardio-toxicity associated with the anthracyclines (Table 54–4).
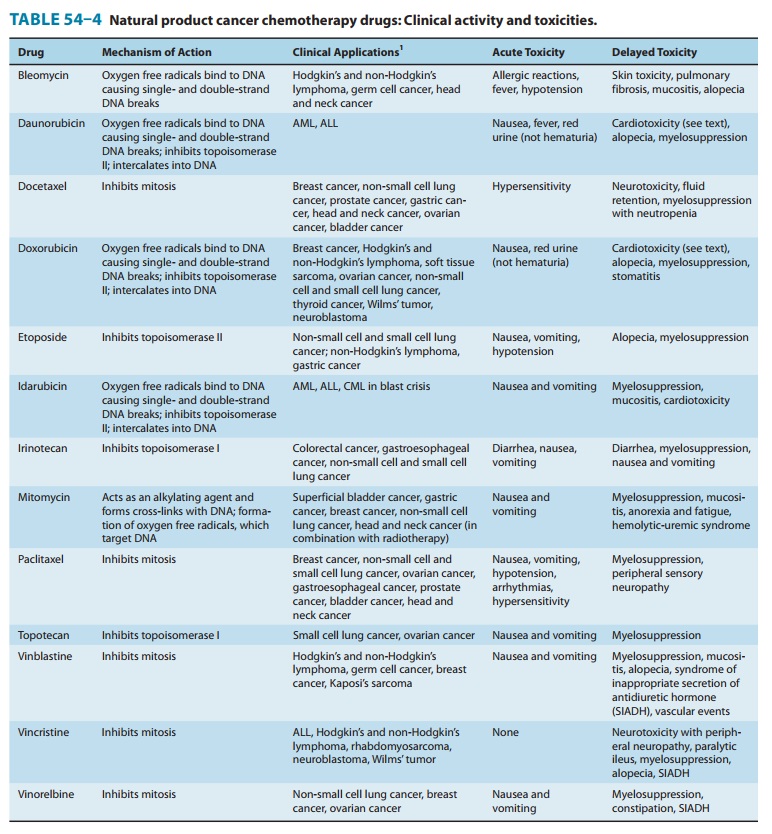
In the clinical setting, anthracyclines are administered via the intravenous route. The anthracyclines are metabolized extensively in the liver, with reduction and hydrolysis of the ring substituents. The hydroxylated metabolite is an active species, whereas the agly-cone is inactive. Up to 50% of drug is eliminated in the feces via biliary excretion, and dose reduction is required in the setting of liver dysfunction. Although anthracyclines are usually administered on an every-3-week schedule, alternative schedules such as low-dose weekly or 72- to 96-hour continuous infusions have been shown to yield equivalent clinical efficacy with reduced toxicity.
Doxorubicin is one of the most important anticancer drugs inclinical practice, with major clinical activity in cancers of the breast, endometrium, ovary, testicle, thyroid, stomach, bladder, liver, and lung; in soft tissue sarcomas; and in several childhood cancers, including neuroblastoma, Ewing’s sarcoma, osteosarcoma, and rhabdomyosarcoma. It also has clinical activity in hematologic malignancies, including acute lymphoblastic leukemia, multiple myeloma, and Hodgkin’s and non-Hodgkin’s lymphomas. It is gen-erally used in combination with other anticancer agents (eg, cyclo-phosphamide, cisplatin, and 5-FU), and clinical activity is improved with combination regimens as opposed to single-agent therapy.
Daunorubicin was the first agent in this class to be isolated,and it is still used in the treatment of acute myeloid leukemia. In contrast to doxorubicin, its efficacy in solid tumors is limited.
Idarubicin is a semisynthetic anthracycline glycoside analog ofdaunorubicin, and it is approved for use in combination with cytarabine for induction therapy of acute myeloid leukemia. When combined with cytarabine, idarubicin appears to be more active than daunorubicin in producing complete remissions and in improving survival in patients with acute myelogenous leukemia.
Epirubicin is an anthracycline analog whose mechanism ofaction and clinical pharmacology are identical to those of all other anthracyclines. It was initially approved for use as a compo-nent of adjuvant therapy in early-stage, node-positive breast cancer but is also used in the treatment of metastatic breast cancer and gastroesophageal cancer.
Mitoxantrone (dihydroxyanthracenedione) is an anthracenecompound whose structure resembles the anthracycline ring. It binds to DNA to produce strand breakage and inhibits both DNA and RNA synthesis. It is currently used in the treatment of advanced, hormone-refractory prostate cancer and low-grade non-Hodgkin’s lymphoma. It is also indicated in breast cancer and in pediatric and adult acute myeloid leukemias. Myelosuppression with leukopenia is the dose-limiting toxicity, and mild nausea and vomiting, mucositis, and alopecia also occur. Although the drug is thought to be less cardiotoxic than doxorubicin, both acute and chronic cardiac toxicity are reported. A blue discoloration of the fingernails, sclera, and urine is observed 1–2 days after drug administration.
The main dose-limiting toxicity of all anthracyclines is myelo-suppression, with neutropenia more commonly observed than thrombocytopenia. In some cases, mucositis is dose-limiting. Two forms of cardiotoxicity are observed. The acute form occurs within the first 2–3 days and presents as arrhythmias and conduction abnormalities, other electrocardiographic changes, pericarditis, and myocarditis. This form is usually transient and in most cases is asymptomatic. The chronic form results in a dose-dependent, dilated cardiomyopathy associated with heart failure. The chronic cardiac toxicity appears to result from increased production of free radicals within the myocardium. This effect is rarely seen at total doxorubicin dosages below 500–550 mg/m2. Use of lower weekly doses or continuous infusions of doxorubicin appear to reduce the incidence of cardiac toxicity. In addition, treatment with the iron-chelating agentdexrazoxane (ICRF-187) is currently approved to prevent or reduce anthracycline-induced cardiotoxicity in women with metastatic breast cancer who have received a total cumulative dose of doxorubicin of 300 mg/m2. The anthracyclines can also produce a “radiation recall reaction,” with erythema and desqua-mation of the skin observed at sites of prior radiation therapy.
MITOMYCIN
Mitomycin (mitomycin C) is an antibiotic isolated from Streptomyces caespitosus. It undergoes metabolic activation throughan enzyme-mediated reduction to generate an alkylating agent that cross-links DNA. Hypoxic tumor stem cells of solid tumors exist in an environment conducive to reductive reactions and are more sensitive to the cytotoxic effects of mitomycin than normal cells and oxygenated tumor cells. It is active in all phases of the cell cycle, and is the best available drug for use in combination with radiation therapy to attack hypoxic tumor cells. Its main clinical use is in the treatment of squamous cell cancer of the anus in combination with 5-FU and radiation therapy. In addition, it is used in combination chemotherapy for squamous cell carcinoma of the cervix and for breast, gastric, and pancreatic cancer. One special application of mitomycin has been in the intravesical treat-ment of superficial bladder cancer. Because virtually none of the agent is absorbed systemically, there is little to no systemic toxicity when used in this setting.
The common toxicities of mitomycin are outlined in Table 54–4. Hemolytic-uremic syndrome, manifested as microan-giopathic hemolytic anemia, thrombocytopenia, and renal failure, as well as occasional instances of interstitial pneumonitis have been reported.
BLEOMYCIN
Bleomycin is a small peptide that contains a DNA-binding region and an iron-binding domain at opposite ends of the molecule. It acts by binding to DNA, which results in single- and double-strand breaks following free radical formation, and inhibition of DNA biosynthesis. The fragmentation of DNA is due to oxida-tion of a DNA-bleomycin-Fe(II) complex and leads to chromo-somal aberrations. Bleomycin is a cell cycle-specific drug that causes accumulation of cells in the G2 phase of the cell cycle.
Bleomycin is indicated for the treatment of Hodgkin’s and non-Hodgkin’s lymphomas, germ cell tumor, head and neck can-cer, and squamous cell cancer of the skin, cervix, and vulva. One advantage of this agent is that it can be given subcutaneously, intramuscularly, or intravenously. Elimination of bleomycin ismainly via renal excretion, and dose modification is recommended in patients with renal dysfunction.
Pulmonary toxicity is dose-limiting for bleomycin and usually presents as pneumonitis with cough, dyspnea, dry inspiratory crackles on physical examination, and infiltrates on chest X-ray. The incidence of pulmonary toxicity is increased in patients older than 70 years of age, in those who receive cumulative doses greater than 400 units, in those with underlying pulmonary disease, and in those who have received prior mediastinal or chest irradiation. In rare cases, pulmonary toxicity can be fatal. Other toxicities are listed in Table 54–4.
Related Topics