Chapter: Basic & Clinical Pharmacology : Cancer Chemotherapy
Nonclassic Alkylating Agents
NONCLASSIC ALKYLATING AGENTS
Several other
compounds have mechanisms of action that involve DNA alkylation as their
cytotoxic mechanism of action. These agents include procarbazine, dacarbazine,
and bendamus-tine. Their respective clinical activities and associated
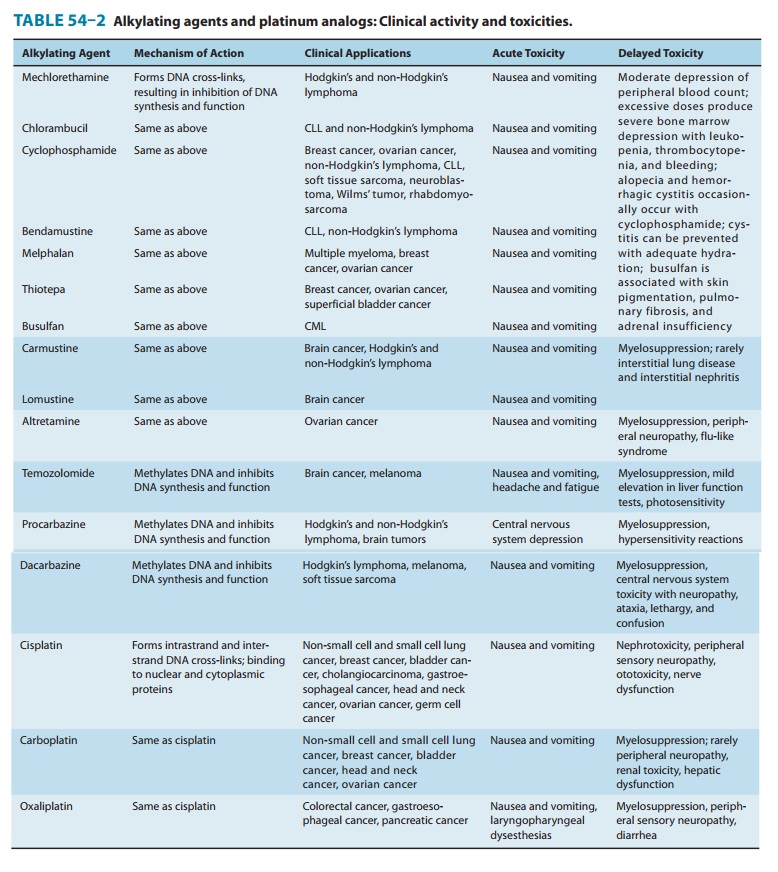
toxicities are listed in Table 54–2.
Procarbazine
Procarbazine is an
orally active methylhydrazine derivative, and in the clinical setting, it is
used in combination regimens for Hodgkin’s and non-Hodgkin’s lymphoma as well
as brain tumors.
The precise mechanism
of action of procarbazine is uncertain; however, it inhibits DNA, RNA, and
protein biosynthesis; pro-longs interphase; and produces chromosome breaks.
Oxidative metabolism of this drug by microsomal enzymes generates
azopro-carbazine and H2O2, which may be responsible for DNA strand scission. A variety of
other drug metabolites are formed that may be cytotoxic. One metabolite is a
weak monoamine oxidase (MAO) inhibitor, and adverse events can occur when
procarbazine is given with other MAO inhibitors as well as with sympathomimetic
agents, tricyclic antidepressants, antihistamines, central nervous system
depressants, antidiabetic agents, alcohol, and tyramine-containing foods.
There
is an increased risk of secondary cancers in the form of acute leukemia, and
its carcinogenic potential is thought to be higher than that of most other
alkylating agents.
Dacarbazine
Dacarbazine
is a synthetic compound that functions as an alkylat-ing agent following
metabolic activation in the liver by oxidative N-demethylation to the monomethyl derivative. This
metabolitespontaneously decomposes to diazomethane, which generates a methyl
carbonium ion that is believed to be the key cytotoxic spe-cies. Dacarbazine is
administered parenterally and is used in the treatment of malignant melanoma,
Hodgkin’s lymphoma, soft tis-sue sarcomas, and neuroblastoma. In terms of safety
profile, the main dose-limiting toxicity is myelosuppression, but nausea and
vomiting can be severe in some cases. This agent is a potent vesi-cant, and
care must be taken to avoid extravasation during drug administration.
Bendamustine
Bendamustine is a
bifunctional alkylating agent consisting of a purine benzimidazole ring and a
nitrogen mustard moiety. As with other alkylating agents, it forms cross-links
with DNA resulting in single- and double-stranded breaks, leading to inhibition
of DNA synthesis and function. This molecule also inhibits mitotic check-points
and induces mitotic catastrophe, which leads to cell death. Of note, the
cross-resistance between bendamustine and other alkylating agents is only
partial, thereby providing a rationale for its clinical activity despite the
development of resistance to other alkylating agents. This agent is approved
for use in chronic lym-phocytic leukemia, with activity also observed in
Hodgkin’s and non-Hodgkin’s lymphoma, multiple myeloma, and breast cancer. The
main dose-limiting toxicities include myelosuppression and mild nausea and
vomiting. Hypersensitivity infusion reactions, skin rash, and other skin
reactions occur rarely.
Related Topics