Chapter: Basic & Clinical Pharmacology : Cancer Chemotherapy
Miscellaneous Anticancer Drugs
MISCELLANEOUS ANTICANCER DRUGS
A
large number of anticancer drugs that do not fit traditional categories have
been approved for clinical use by the Food and Drug Administration (FDA); they
are listed in Table 54–5.
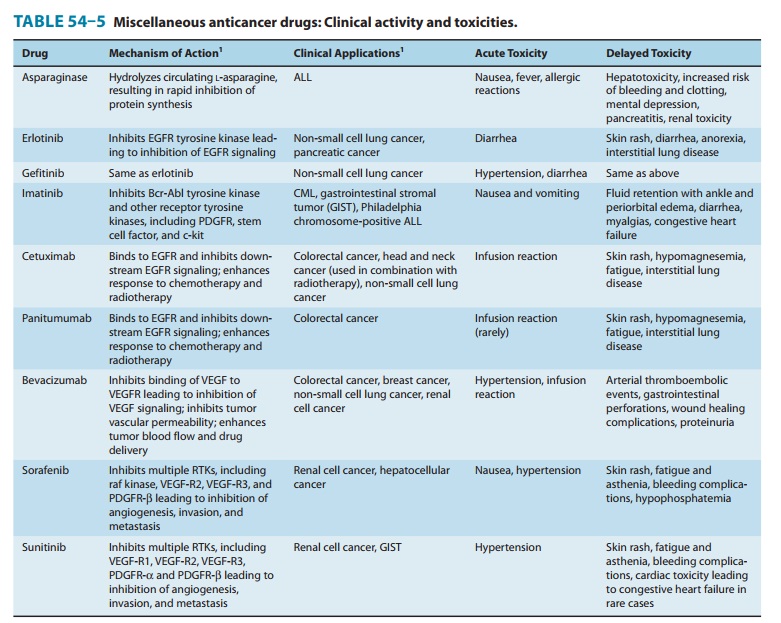
IMATINIB, DASATINIB, & NILOTINIB
Imatinib
is an inhibitor of the tyrosine kinase domain of the Bcr-Abl oncoprotein and
prevents phosphorylation of the kinase sub-strate by ATP. It is indicated for
the treatment of chronic myelogenous leukemia (CML), a pluripotent
hematopoietic stem cell disorder characterized by the t(9:22) Philadelphia
chromo-somal translocation. This translocation results in the Bcr-Abl fusion
protein, the causative agent in CML, and is present in up to 95% of patients
with this disease. This agent also inhibits other receptor tyrosine kinases for
platelet-derived growth factor recep-tor (PDGFR), stem cell factor, and c-kit.
Imatinib is well
absorbed orally, and it is metabolized in the liver, with elimination of
metabolites occurring mainly in feces via biliary excretion. This agent is
approved for use as first-line ther-apy in chronic phase CML, in blast crisis,
and as second-line therapy for chronic phase CML that has progressed on prior
interferon-alfa therapy. Imatinib is also effective in the treatment of
gastrointestinal stromal tumors expressing the c-kit tyrosine kinase. The main
adverse effects are listed in Table 54–5.
Dasatinib is an oral
inhibitor of several tyrosine kinases, includ-ing Bcr-Abl, Src, c-kit, and
PDGFR-α.
It differs from imatinib in that it binds to the active and inactive conformations
of the Abl kinase domain and overcomes imatinib resistance resulting from
mutations in the Bcr-Abl kinase. It is approved for use in CML and Philadelphia
chromosome-positive acute lymphoblastic leukemia (ALL) with resistance or
intolerance to imatinib therapy.
Nilotinib is a second-generation phenylamino-pyrimidine mol-ecule that inhibits Bcr-Abl, c-kit, and PDGFR-β tyrosine kinases. It has a higher binding affinity (up to 20- to 50-fold) for the Abl kinase when compared with imatinib, and it overcomes imatinib resistance resulting from Bcr-Abl mutations. It was originally approved for chronic phase and accelerated phase CML with resis-tance or intolerance to prior therapy that included imatinib and was recently approved as first-line therapy of chronic phase CML.
Imatinib, dasatinib,
and nilotinib are all metabolized in the liver, mainly by the CYP3A4 liver
microsomal enzyme. A large fraction of each drug is eliminated in feces via the
hepatobiliary route. It is important to review the patient’s current list of
prescription and nonprescription drugs because these agents have potential
drug-drug interactions, especially with those that are also metabolized by the
CYP3A4 system. In addition, patients should avoid grapefruit products and the
use of St. John’s wort, as they may alter the metabolism of these small
molecule inhibitors .
GROWTH FACTOR RECEPTOR INHIBITORS
Cetuximab & Panitumumab
The
epidermal growth factor receptor (EGFR) is a member of the erb-B family of
growth factor receptors, and it is overexpressed in a number of solid tumors,
including colorectal cancer, head and neck cancer, non-small cell lung cancer,
and pancreatic cancer. Activation of the EGFR signaling pathway results in
downstream activation of several key cellular events involved in cellular
growth and prolifera-tion, invasion and metastasis, and angiogenesis. In
addition, this pathway inhibits the cytotoxic activity of various anticancer
agents and radiation therapy, presumably through suppression of key apoptotic
mechanisms, thereby leading to the development of cel-lular drug resistance.
Cetuximab is a
chimeric monoclonal antibody directed against the extracellular domain of the
EGFR, and it is presently approved for use in combination with irinotecan for
metastatic colon cancer in the refractory setting or as monotherapy in patients
who are deemed to be irinotecan-refractory. Because cetuximab is of the G 1 isotype, its
antitumor activity may also be mediated, in part, by immunologic-mediated
mechanisms. There is growing evi-dence that cetuximab can be effectively and
safely combined with irinotecan- and oxaliplatin-based chemotherapy in the
first-line treatment of metastatic colorectal cancer as well. Of note, the
efficacy of cetuximab is restricted to only those patients whose tumors express
wild-type KRAS. Regimens combining
cetuximab with cytotoxic chemotherapy may be of particular benefit in the
neoadjuvant therapy of patients with liver-limited disease. Although this antibody
was initially approved to be administered on a weekly schedule, pharmacokinetic
studies have shown that an every-2-week schedule provides the same level of
clinical activity as the weekly schedule. This agent is also approved for use
in combination with radiation therapy in patients with locally advanced head
and neck cancer. Cetuximab is well tolerated, with the main adverse effects
being an acneiform skin rash, hypersensi-tivity infusion reaction, and
hypomagnesemia.
Panitumumab
is a fully human monoclonal antibody directed against the EGFR and works
through inhibition of the EGFR signaling pathway. In contrast to cetuximab,
this antibody is of the G2 isotype,
and as such, it would not be expected to exert any immunologic-mediated
effects. Presently, panitumumab is approved for patients with refractory
metastatic colorectal cancer who have been treated with all other active
agents, and as with cetuximab, this antibody is only effective in patients
whose tumors express wild-type KRAS.
Recent clinical studies have shown that this anti-body is effectively and
safely combined with oxaliplatin- and irino-tecan-based chemotherapy in the
first- and second-line treatment of metastatic colorectal cancer. Acneiform
skin rash and hypomag-nesemia are the two main adverse effects associated with
its use. Because this is a fully human antibody, infusion-related reactions are
rarely observed.
Gefitinib & Erlotinib
Gefitinib and
erlotinib are small molecule inhibitors of the tyrosine kinase domain
associated with the EGFR, and both are used in the treatment of non-small cell
lung cancer that is refractory to at least one prior chemotherapy regimen.
Patients who are nonsmokers and who have a bronchoalveolar histologic subtype
appear to be more responsive to these agents. In addition, erlotinib has been
approved for use in combination with gemcitabine for the treat-ment of advanced
pancreatic cancer. Both agents are metabolized in the liver by the CYP3A4
enzyme system, and elimination is mainly hepatic with excretion in feces.
Caution must be taken when using these agents with drugs that are also
metabolized by the liver CYP3A4 system, such as phenytoin and warfarin, and the
use of grapefruit products should be avoided. An acneiform skin rash, diarrhea,
and anorexia and fatigue are the most common adverse effects observed with
these small molecules (Table 54-5).
Bevacizumab, Sorafenib, Sunitinib, & Pazopanib
The vascular
endothelial growth factor (VEGF) is one of the most important angiogenic growth
factors. The growth of both primary and metastatic tumors requires an intact
vasculature. As a result, the VEGF-signaling pathway represents an attractive
target for chemo-therapy. Several approaches have been taken to inhibit VEGF
sig-naling; they include inhibition of VEGF interactions with its receptor by
targeting either the VEGF ligand with antibodies or soluble chimeric decoy
receptors, or by direct inhibition of the VEGF receptor-associated tyrosine
kinase activity by small mole-cule inhibitors.
Bevacizumab is a
recombinant humanized monoclonal anti-body that targets all forms of VEGF-A.
This antibody binds to and prevents VEGF-A from interacting with the target
VEGF receptors. Bevacizumab can be safely and effectively combined with 5-FU-,
irinotecan-, and oxaliplatin-based chemotherapy in the treatment of metastatic
colorectal cancer. Bevacizumab is FDA approved as a first-line treatment for
metastatic colorectal cancer in combination with any intravenous
fluoropyrimidine-contain-ing regimen and is now also approved in combination
with che-motherapy for metastatic non-small lung cancer and breast cancer. One
potential advantage of this antibody is that it does not appear to exacerbate
the toxicities typically observed with cytotoxic che-motherapy. The main safety
concerns associated with bevacizumab include hypertension, an increased
incidence of arterial throm-boembolic events (transient ischemic attack, stroke,
angina, and myocardial infarction), wound healing complications,
gastrointes-tinal perforations, and proteinuria.
Sorafenib
is a small molecule that inhibits multiple receptor tyrosine kinases (RTKs),
especially VEGF-R2 and VEGF-R3, platelet-derived growth factor-β (PDGFR-β), and raf
kinase. It was initially approved for advanced renal cell cancer and is also
approved for advanced hepatocellular cancer.
Sunitinib is similar to sorafenib in that it inhibits multiple RTKs, although the specific types are somewhat different. They include PDGFR-α and PDGFR-β, VEGF-R1, VEGF-R2, VEGF-R3, and c-kit. It is approved for the treatment of advanced renal cell cancer and for the treatment of gastrointestinal stromal tumors (GIST) after disease progression on or with intolerance to imatinib.
Pazopanib
is a small molecule that inhibits multiple RTKs, espe-cially VEGF-R2 and
VEGF-R3, PDGFR-β,
and raf kinase. This oral agent is approved for the treatment of advanced renal
cell cancer.
Sorafenib, sunitinib,
and pazopanib are metabolized in the liver by the CYP3A4 system, and
elimination is primarily hepatic with excretion in feces. Each of these agents
has potential interac-tions with drugs that are also metabolized by the CYP3A4
system, especially warfarin. In addition, patients should avoid grapefruit
products and the use of St. John’s wort, as they may alter the clinical
activity of these agents. Hypertension, bleeding complica-tions, and fatigue
are the most common adverse effects seen with both agents. With respect to sorafenib,
skin rash and the hand-foot syndrome are observed in up to 30–50% of patients.
For sunitinib, there is also an increased risk of cardiac dysfunction, which in
some cases can lead to congestive heart failure.
ASPARAGINASE
Asparaginase (L-asparagine
amidohydrolase) is an enzyme used to treat childhood ALL. The drug is isolated
and purified from Escherichia coli or Erwinia chrysanthemi for clinical use.
It hydro-lyzes circulating L-asparagine
to aspartic acid and ammonia. Because tumor cells in ALL lack asparagine
synthetase, they require an exogenous source of L-asparagine. Thus, depletion of L-asparagine results in effective inhibition of protein
synthesis. In contrast,
normal cells can synthesize L-asparagine
and thus are less susceptible to the cytotoxic action of asparaginase. The main
adverse effect of this agent is a hypersensitivity reaction manifested by
fever, chills, nausea and vomiting, skin rash, and urticaria. Severe cases can
present with bronchospasm, respiratory failure, and hypotension. Other side
effects include an increased risk of both clotting and bleeding as a result of
alterations in various clot-ting factors, pancreatitis, and neurologic toxicity
with lethargy, confusion, hallucinations, and in severe cases, coma.
Related Topics