Chapter: Biochemistry: Carbohydrates
Sugars: Their Structures and Stereochemistry
Sugars: Their Structures and
Stereochemistry
When the
word carbohydrate was coined, it
originally referred to compounds of the general formula Cn(H2O)n.
However, only the simple sugars, or monosaccharides,
fit this formula exactly. The other types of carbohydrates,oligosaccharides
and polysaccharides, are based on the monosaccharide units and have slightly
different general formulas. Oligosaccharides
are formed when a few (Greek oligos)
monosaccharides are linked; polysaccharides are formed when many (Greek polys) monosaccharides are bonded
together. The reaction that adds monosaccharide units to a growing carbohydrate
molecule involves the loss of one H2O for each new link formed,
accounting for the difference in the general formula.
Many
commonly encountered carbohydrates are polysaccharides, including glycogen,
which is found in animals, and starch and cellulose, which occur in plants.
Carbohydrates play a number of important roles in biochemistry. First, they are
major energy. Second, oligosaccharides play a key role in processes that take
place on the surfaces of cells, particularly in cell–cell interactions and
immune recognition. In addition, polysaccharides are essential structural
components of several classes of organisms. Cellulose is a major component of
grass and trees, and other polysaccharides are major components of bacterial
cell walls.
What is unique about the structures of sugars?
The
building blocks of all carbohydrates are the simple sugars called monosaccharides. A monosaccharide can
be a polyhydroxy aldehyde (aldose) or
a polyhydroxy ketone (ketose). The
simplest monosaccharides contain three carbon atoms and are called trioses (tri meaning “three”). Glyceraldehyde is the aldose with three
carbons (an aldotriose), and dihydroxyacetone
is the ketose with three carbon atoms (a ketotriose). Figure 16.1 shows these
molecules.
Aldoses
with four, five, six, and seven carbon atoms are called aldotetroses,
aldopentoses, aldohexoses, and aldoheptoses, respectively. The corresponding
ketoses are ketotetroses, ketopentoses, ketohexoses, and ketoheptoses.
Six-carbon sugars are the most abundant in nature, but two five-carbon sugars,
ribose and deoxyribose, occur in the structures of RNA and DNA, respectively.
Four-carbon and seven-carbon sugars play roles in photosynthesis and other
metabolic pathways.
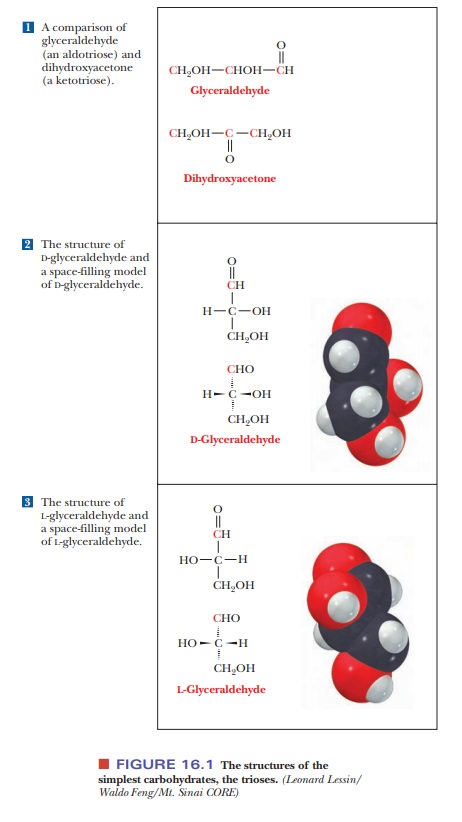
We have already seen that some molecules are not super-imposable on their mirror images and that these mirror images are opticalisomers (stereoisomers) of each other. A chiral (asymmetric) carbon atom isthe usual source of optical isomerism, as was the case with amino acids. The simplest carbohydrate that contains a chiral carbon is glyceraldehyde, which can exist in two isomeric forms that are mirror images of each other [Figure 16.1(2) and (3)].
Note that the two forms differ in the position of the
hydroxyl group bonded to the central carbon. (Dihydroxyacetone does not contain
a chiral carbon atom and does not exist in nonsuperimposable mirror-image
forms.) The two forms of glyceraldehyde are designated D-glyceraldehyde and
L-glyceraldehyde. Mirror-image stereoisomers are also calledenantiomers,and D-glyceraldehyde and
L-glyceraldehyde are enantiomers of each other. Certainconventions are used for
two-dimensional drawings of the three-dimensional structures of stereoisomers.
The dashed wedges represent bonds directed away from the viewer, below the
plane of the paper, and the solid wedges represent bonds directed oppositely,
toward the viewer and out of the plane of the paper. The configuration is the three-dimensional arrangement of groups around
a chiral carbon atom, and stereoisomers differ from each other in
configura-tion. The D,L system to
denote stereochemistry is widely used by biochemists. Organic chemists tend to
use a more recent one, designated the R,S
system. There is not a one-to-one correspondence between the two systems. For
exam-ple, some D-isomers are R, and
some are S.
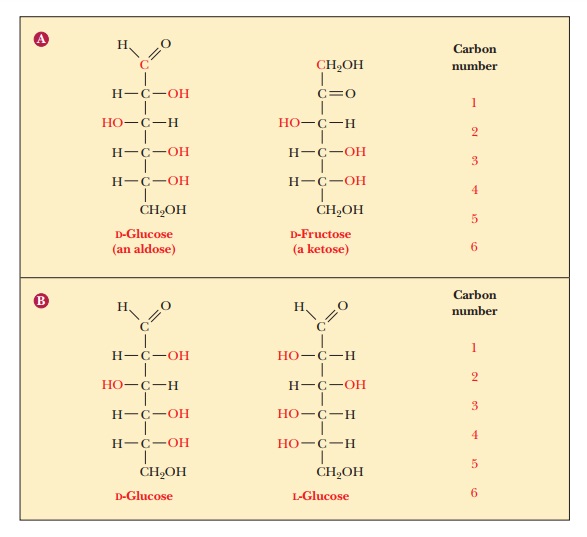
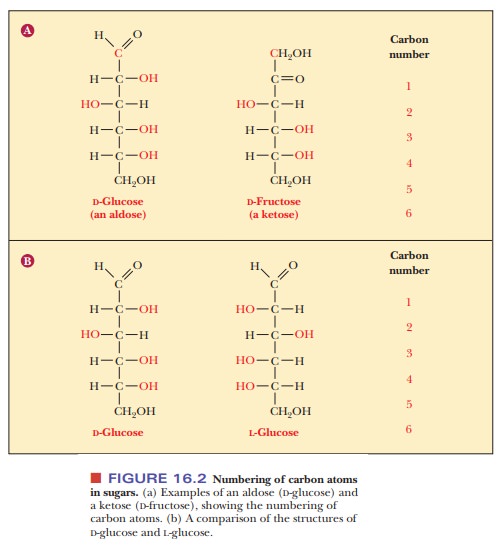
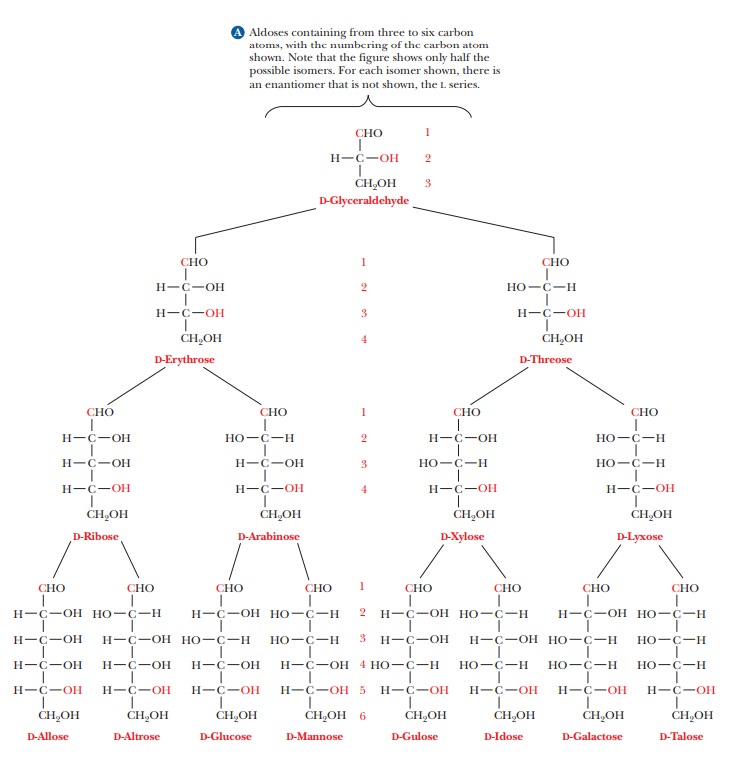
The two enantiomers of glyceraldehyde are the only possible stereoisomers of three-carbon sugars, but the possibilities for stereoisomerism increase as the number of carbon atoms increases. To show the structures of the resulting molecules, we need to say more about the convention for a two-dimensional perspective of the molecular structure, which is called the Fischer projection method, after the German chemist Emil Fischer, who established the structures of many sugars. We shall use some common six-carbon sugars for purposes of illustration. In a Fischer projection, bonds written vertically on the two-dimensional paper represent bonds directed behind the paper in three dimen-sions, whereas bonds written horizontally represent bonds directed in front of the paper in three dimensions. Figure 16.2 shows that the most highly oxidized carbon—in this case, the one involved in the aldehyde group—is written at the “top” and is designated carbon 1, or C-1. In the ketose shown, the ketone group becomes C-2, the carbon atom next to the “top.” Most common sugars are aldoses rather than ketoses, so our discussion will focus mainly on aldoses. The other carbon atoms are numbered in sequence from the “top.” The desig-nation of the configuration as L or D depends on the arrangement at the chiral carbon with the highest number. In the cases of both glucose and fructose, this is C-5. In the Fischer projection of the D configuration, the hydroxyl group is on the right of the highest-numbered chiral carbon, whereas the hydroxyl group is on the left of the highest-numbered chiral carbon in the L configuration. Let us see what happens as another carbon is added to glyceraldehyde to give a four-carbon sugar. In other words, what are the possible stereoisomers for an aldotetrose? The aldotetroses (Figure 16.3) have two chiral carbons, C-2 and C-3, and there are 22, or four, possible stereoisomers.
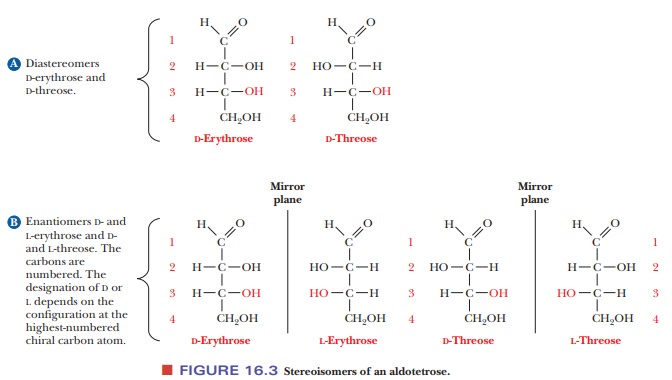
Two of the isomers have the D
configuration, and two have the L configuration. The two D isomers have the
same configuration at C-3, but they differ in configuration (arrangement of the
—OH group) at the other chiral carbon, C-2. These two isomers are called
D-erythrose and D-threose. They are not superimposable on each other, but
nei-ther are they mirror images of each other. Such nonsuperimposable,
non-mirror-image stereoisomers are called diastereomers.
The two L isomers are L-erythrose and L-threose. L-Erythrose is the enantiomer
(mirror image) of D-erythrose, and L-threose is the enantiomer of D-threose.
L-Threose is a diastereomer of both D- and L-erythrose, and L-erythrose is a
diastereomer of both D- and L-threose.Diastereomers that differ from each other
in the configuration at only one chiral carbon are called epimers;D-erythrose and D-threose are epimers.
Aldopentoses
have three chiral carbons, and there are 23, or 8, possible
stereoisomers—four D forms and four L forms. Aldohexoses have four chiral
carbons and 24, or 16, stereoisomers—eight D forms and eight L forms
(Figure 16.4). Some of the possible stereoisomers are much more common in
nature than others, and most biochemical discussion centers on the common,
nat-urally occurring sugars. For example, D sugars, rather than L sugars,
predomi-nate in nature. Most of the sugars we encounter in nature, especially
in foods, contain either five or six carbon atoms. We shall discuss D-glucose
(an aldohex-ose) and D-ribose (an aldopentose) far more than many other sugars.
Glucose is a ubiquitous energy source, and ribose plays an important role in
the struc-ture of nucleic acids.

What happens if a sugar forms a cyclic molecule?
Sugars, especially those with five or six carbon atoms, normally exist as cyclic molecules rather than as the open-chain forms we have shown so far. The cyclization takes place as a result of interaction between the functional groups on distant carbons, such as C-1 and C-5, to form a cyclic hemiacetal (in aldohexoses). Another possibility (Figure 16.5) is interaction between C-2 and C-5 to form a cyclic hemiketal (in ketohexoses). In either case, the carbonyl carbon becomes a new chiral center called the anomeric carbon. The cyclic sugar can take either of two different forms, designated α and β, and are called anomers of each other.
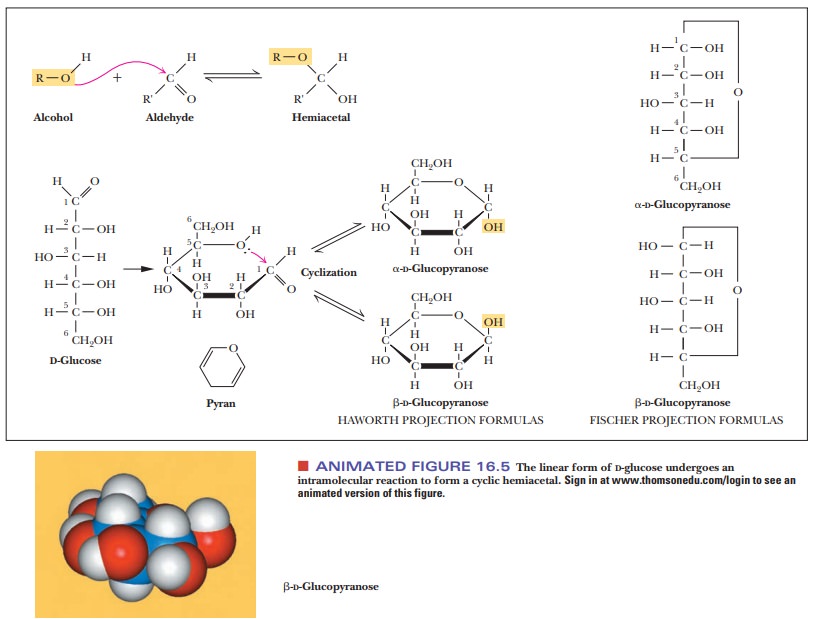
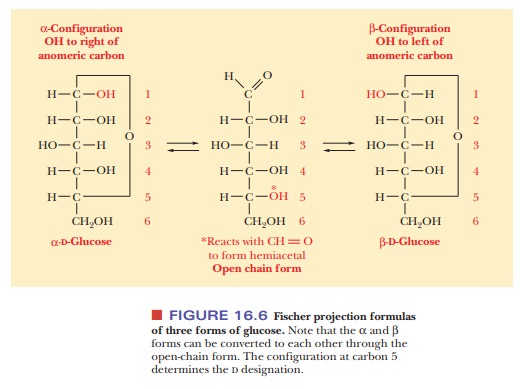
The
Fischer projection of the α-anomer of a D sugar has the anomeric hydroxyl group
to the right of the anomeric carbon (C—OH), and the β-anomer of aDsugar has the anomeric hydroxyl group to the left of
theanomeric carbon (Figure 16.6). The free carbonyl species can readily form
either the α- or β-anomer, and the anomers can be converted from
one form to another through the free carbonyl species. In some biochemical
molecules, any anomer of a given sugar can be used, but, in other cases, only
one anomer occurs. For example, in living organisms, only β-D-ribose and β-D-deoxyribose are found in RNA and DNA,
respectively.
Fischer projection formulas are useful for describing the stereochemistry of sugars, but their long bonds and right-angle bends do not give a realistic picture of the bonding situation in the cyclic forms, nor do they accurately represent the overall shape of the molecules. Haworth projection formulas are more useful for those purposes. In Haworth projections, the cyclic structures of sugars are shown in perspective drawings as planar five- or six-membered rings viewed nearly edge on. A five-membered ring is called a furanose because of its resemblance to furan; a six-membered ring is called a pyranose because of its resemblance to pyran [Figure 16.7(a) and (b)].
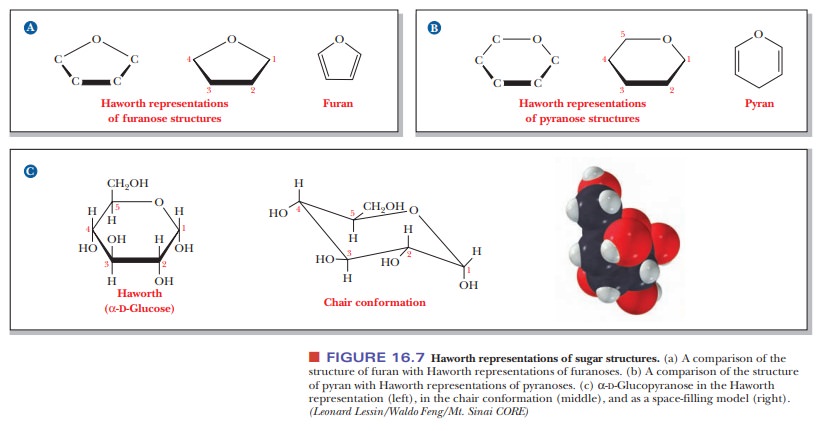
These cyclic formulas approximate the shapes of the actual molecules
better for furanoses than for pyranoses. The five-membered rings of furanoses
are in reality very nearly pla-nar, but the six-membered rings of pyranoses
actually exist in solution in the chair conformation [Figure 16.7(c)]. This
kind of structure is particularly use-ful in discussions of molecular
recognition. The chair conformation and the Haworth projections are alternative
ways of expressing the same information. Even though the Haworth formulas are
approximations, they are useful short-hand for the structures of reactants and
products in many reactions that we are going to see. The Haworth projections
represent the stereochemistry of sugars more realistically than do the Fischer
projections, and the Haworth scheme is adequate for our purposes. That is why
biochemists use them, even though organic chemists prefer the chair form. We
shall continue to use Haworth pro-jections in our discussion of sugars.
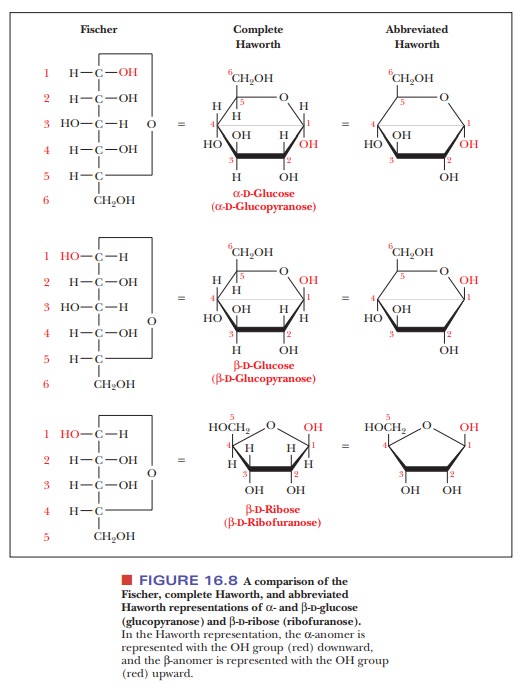
For a D
sugar, any group that is written to the right
of the carbon in a Fischer projection has a downward
direction in a Haworth projection; any group that is written to the left in a Fischer projection has an upward direction in a Haworth
projection. The terminal —CH2OH group, which contains the carbon
atom with the highest number in the numbering scheme, is shown in an upward
direction. The structures of α- and β-D-glucose, which are both pyranoses, and of β-D-ribose, which is a furanose, illustrate this point (Figure
16.8). Note that, in the α-anomer, the hydroxyl on the anomeric carbon is
on the opposite side of the ring from the terminal —CH2OH group
(i.e., pointing down). In the β-anomer, it is on the same side of the ring
(pointing up). The same conven-tion holds for α- and β-anomers of furanoses.
Summary
Biochemically important sugars usually contain five or six carbon
atoms; their structure includes a carbonyl group (either the aldehyde or the ketone
form) and several hydroxyl groups.
Optical isomerism is of paramount importance in the structure of
simple sugars. Most of the important sugars found in nature have the D configu-ration, based on the standard compound D-glyceraldehyde.
Most sugars exist in cyclic forms with five- or six-membered rings.
The cyclization process involves the carbonyl group and gives rise to another
chiral center in addition to the ones already present in the sugar mol-ecule.
The
Related Topics