Chapter: Biochemistry: Carbohydrates
Reactions of Monosaccharides
Reactions of Monosaccharides
What are some oxidation–reduction reactions of sugars?
Oxidation and reduction reactions of sugars play key roles in
biochemistry. Oxidation of sugars provides energy for organisms to carry out their
life processes; the highest yield of energy from carbohydrates occurs when
sugars are completely oxidized to CO2 and H2O in
aerobic processes. The reverse of complete oxidation of sugars is the reduction
of CO2 and H2O to form sugars, a process
that takes place in photosynthesis.
Several oxidation reactions of sugars are of some importance in
laboratory practice because they can be used to identify sugars. Aldehyde
groups can be oxidized to give the carboxyl group that is characteristic of
acids, and this reac-tion is the basis of a test for the presence of aldoses.
When the aldehyde is oxi-dized, some oxidizing agent must be reduced. Aldoses
are called reducing sugars because
of this type of reaction; ketoses can also be reducing sugars because they isomerize
to aldoses. In the cyclic form, the compound produced by oxidation of an aldose
is a lactone (a cyclic ester linking
the carboxyl group and one of the sugar alcohols, as shown in Figure 16.9).
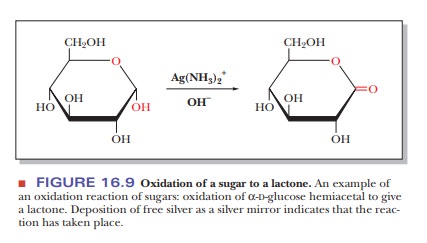
Two types of reagent are used in the laboratory to detect the presence of reducing sugars. The first of these is Tollens reagent, which uses the silver ammonia complex ion, Ag(NH3)2+, as the oxidizing agent. A silver mirror is deposited on the wall of the test tube if a reducing sugar is present, as a result of the Ag+ in the complex ion being reduced to free silver metal (Figure 16.10). A more recent method for detection of glucose, but not other reducing sugars, is based on the use of the enzyme glucose oxidase, which is specific for glucose.

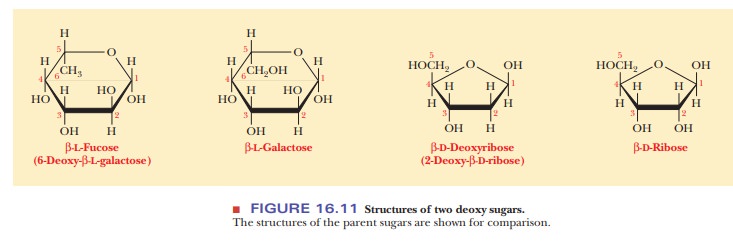
In addition to oxidized sugars, there are some important reduced sugars. In deoxy sugars, a hydrogen atom is substituted for one of the hydroxyl groups of thesugar. One of these deoxy sugars is L-fucose (L-6-deoxygalactose), which is found in the carbohydrate portions of some glycoproteins (Figure 16.11), including the ABO blood-group antigens. The name glycoprotein indicates that these substances are conjugated proteins that contain some carbohydrate group (glykos is Greek for “sweet”) in addition to the polypeptide chain. An even more important exam-ple of a deoxy sugar is D-2-deoxyribose, the sugar found in DNA (Figure 16.11).
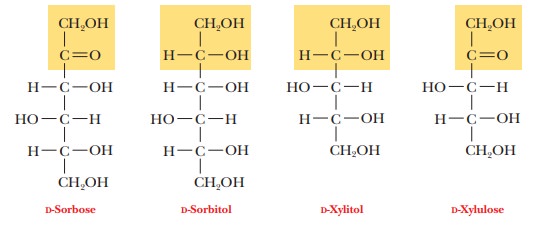
When the carbonyl group of a sugar is reduced to a hydroxyl group, the resulting compound is one of the polyhydroxy alcohols known as alditols. Two compounds of this kind, xylitol and sorbitol, derivatives of the sugars xylulose and sorbose, respectively, have commercial importance as sweeteners in sugar-less chewing gum and candy.
What are some important esterification reactions of sugars?
The hydroxyl groups of sugars behave exactly like all other
alcohols in the sense that they can react with acids and derivatives of acids
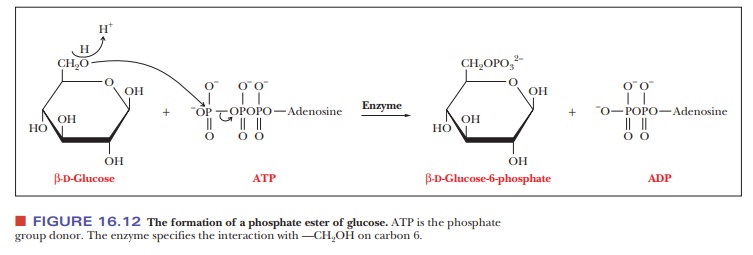
to form esters. The phosphate esters are particularly important because they
are the usual intermediates in the breakdown of carbohydrates to provide
energy. Phosphate esters are frequently formed by transfer of a phosphate group
from ATP (adenosine triphosphate) to give the phosphorylated sugar and ADP
(adenosine diphosphate), as shown in Figure 16.12. Such reactions play an
important role in the metabolism of sugars.
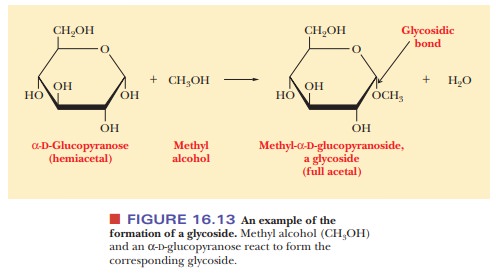
What are glycosides, and how do they form?
It is possible for a sugar hydroxyl group (ROH) bonded to the anomeric carbon to react with another hydroxyl (R'—OH) to form a glycosidic linkage (R'—O— R). A glycosidic linkage is not an ether (the R'—O—R notation is misleading) because glycosides can be hydrolyzed to the original alcohols.
This type of reaction involves the
anomeric carbon of the sugar in its cyclic form. (Recall that the anomeric
carbon is the carbonyl carbon of the open-chain form of the sugar and is the
one that becomes a chiral center in the cyclic form.) Stated in a slightly
different way, a hemiacetal carbon can react with an alcohol such as methyl
alcohol to give a full acetal, or glycoside (Figure 16.13). The newly
formed bond is called a glycosidic bond.
Glycosides derived from furanoses are called furanosides, and those derived from pyranoses are called pyranosides.
Glycosidic bonds between monosaccharide units are the basis for the
for-mation of oligosaccharides and polysaccharides. Glycosidic linkages can
take various forms; the anomeric carbon of one sugar can be bonded to any one
of the —OH groups on a second sugar to form an α- or β-glycosidic linkage. Many different
combinations are found in nature. The —OH groups are numbered so that they can
be distinguished, and the numbering scheme follows that of the carbon atoms.
The notation for the glycosidic linkage between the two sug-ars specifies which
anomeric form of the sugar is involved in the bond and also specifies which
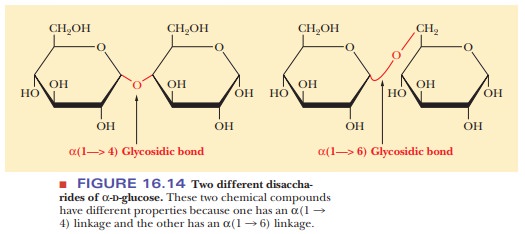
carbon atoms of the two sugars are linked together. Two ways in which two α-D-glucose
molecules can be linked together are α(1 - > 4) and α(136). In the first example, the α-anomeric carbon (C-1) of the
first glucosemolecule is joined in a glycosidic bond to the fourth carbon atom
(C-4) of the second glucose molecule; the C-1 of the first glucose molecule is
linked to the C-6 of the second glucose molecule in the second example (Figure
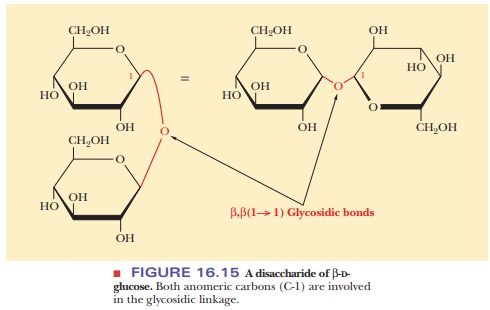
16.14). Another possibility of a glycosidic bond, this time between two β-D-glucose
mol-ecules, is a β,β(1 - > 1) linkage. The
anomeric forms at both C-1 carbons must be specified because the linkage is
between the two anomeric carbons, each of which is C-1 (Figure 16.15).
When oligosaccharides and polysaccharides form as a result of glycosidic bonding, their chemical natures depend on which monosaccharides are linked together and also on the particular glycosidic bond formed (i.e., which anomers and which carbon atoms are linked together).
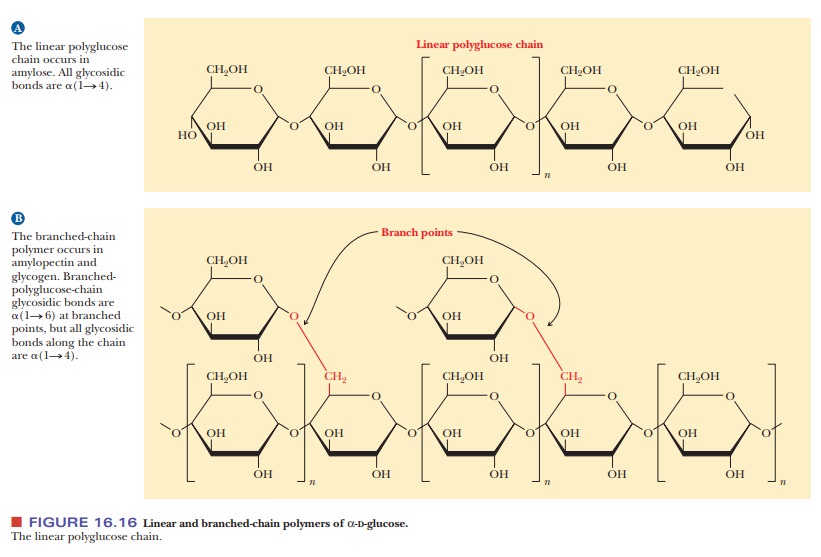
The difference
between cellulose and starch depends on the glycosidic bond formed between
glucose monomers. Because of the variation in glycosidic linkages, both linear
and branched-chain polymers can be formed. If the internal monosaccharide
resi-dues that are incorporated in a polysaccharide form only two glycosidic
bonds, the polymer will be linear. (Of course, the end residues will be
involved in only one glycosidic linkage.) Some internal residues can form three
glycosidic bonds, leading to the formation of branched-chain structures (Figure
16.16).
Another point about glycosides is worth mentioning. We have already
seen that the anomeric carbon is frequently involved in the glycosidic linkage,
and also that the test for the presence of sugars—specifically for reducing
sug-ars—requires a reaction of the group at the anomeric carbon. The internal anomeric
carbons in oligosaccharides are not free to give the test for reduc-ing sugars.
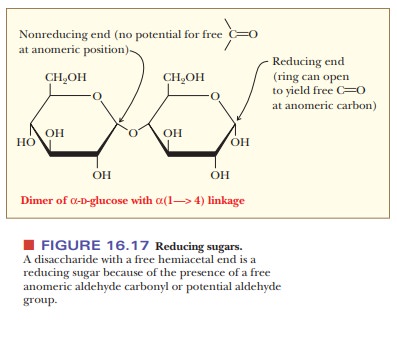
Only if the end residue is a free hemiacetal rather than a glycoside will there
be a positive test for a reducing sugar (Figure 16.17). The level of detection
can be important for such a test. A sample that contains only a few molecules
of a large polysaccharide, each molecule with a single reducing end, might well
produce a negative test because there are not enough reducing ends to detect.
What are some other important derivatives of sugars?
Amino sugars are an interesting class of compounds related to themonosaccharides. We shall not go into the chemistry of their formation, but it will be useful to have some acquaintance with them when we discuss polysaccharides.
In sugars of this type, an amino group (—NH2) or one
of its derivatives is substituted for the hydroxyl group of the parent sugar.
In N-acetyl amino sugars, the amino
group itself carries an acetyl group (CH3—CO—) as
a substituent.
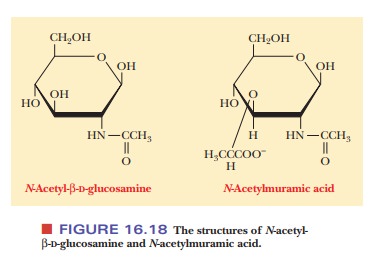
Two particularly important examples are N-acetyl-β-D-glucosamine and its
derivative N-acetyl-β-muramic acid, which has an
added carboxylic acid side chain (Figure 16.18). These two compounds are
components of bacterial cell walls. We did not specify whether N-acetylmuramic acid belongs to the L or the D series of configurations, and we did not
specify theα- orβ-anomer. This typeof
shorthand is the usual practice with β-D-glucose and its derivatives;
the D con-figuration and the β-anomeric form are so common
that we need not specify them all the time unless we want to make some specific
point. The position of the amino group is also left unspecified because
discussion of amino sugars usually centers on a few compounds whose structures
are well known.
Summary
Sugars can and do undergo oxidation reactions, as well as forming
esters.
Glycosidic linkages are responsible for the bonding of
monosaccharides to form oligosaccharides and polysaccharides. The reaction in
question takes place when one sugar hydroxyl group forms a bond with another
sugar hydroxyl, usually one on an anomeric carbon. Different stereo-chemical forms
are possible in glycosidic linkages, having important con-sequences for the
function of the substances thus formed.
Related Topics