Chapter: Biochemistry: Carbohydrates
Physical properties of monosaccharides
Physical properties of
monosaccharides
1. Colour and shape
Monosaccharides are colourless and crystalline
compounds.
2. Solubility
They are readily soluble in water.
3. Taste
They have sweet tase.
4. Stereo isomerism
D-glucose and L-glucose are mirror images of
each other.
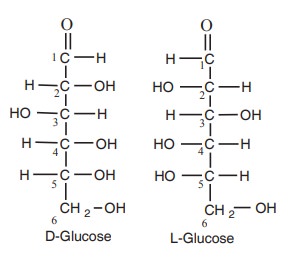
The presence of asymmetric carbon atoms in a
compound give rise to the formation of isomers of that compound. Such compound
which are identical in composition and differs only in spatial configuration
are called “stereo isomers’’. For example glucose can exist in two forms as
shown below.
D-series and L-series
The orientation of the H and OH groups around
the carbon atom just adjacent to the terminal primary alcohol carbon, eg. C5
in glucose determines the series. The D and L forms of glyceraldehyde are given
below.
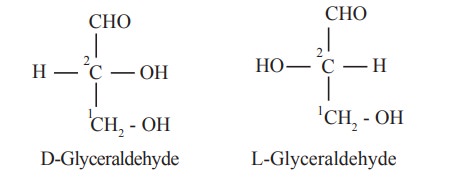
when the -OH group of this C2 is at
the right, it belongs to D-series, when the -OH group is on the left it belongs
to L-series.
5. Optical Isomerism
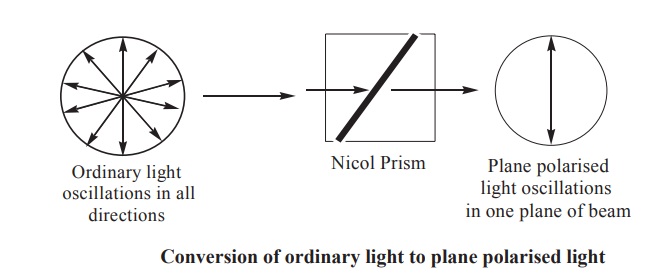
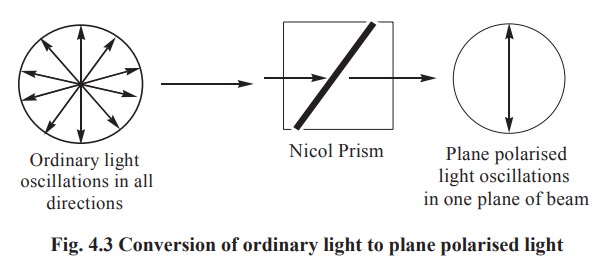
A beam of ordinary light may be regarded as
bundle of electromagnetic waves vibrating in all directions perpendicular to
the axis of the beam. When such a beam of light is made to pass through a nicol
prism, all vibrations except those in one plane are eliminated. This is called
as plane polarised light. When such a beam of plane polarised light is passed
through a solution of an optical isomer, and if the plane polarised light is
found to rotate to the left, it is described as levorotation. If the plane polarised
light rotates to an equal number of degrees to the right, it is described as
dextrorotation. This phenomenon exhibited by asymmetric compounds, is called
optical isomerism (Fig. 4.3 & 4.4).
Polarimeter is an instrument by which the specific
rotations of optical isomers are detected.
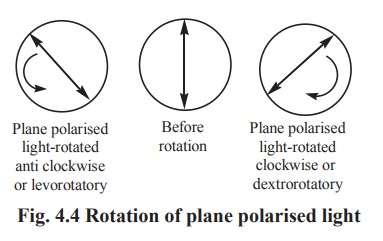
Expression of optical activity
Optical rotation to the left i.e levorotation
is expressed with a sign of l- and rotation to the right i.e dextrorotation is
expressed as d+.
Racemic mixture
When equal amounts of dextrorotatory and
levorotatory isomers are present, the resulting mixture has no optical
activity, since the activities of isomers cancel each other. Such a mixture is
said to be a “racemic mixture”.
Resolution
The separation of optically active isomers from
a racemic mixture is called resolution.
6. Mutarotation
When an aldohexose is first dissolved in water
and the solution is kept in optical path and plane polarised light is passed,
the initial optical rotation shown by the sugar gradually changes until a
constant fixed rotation characteristic of the sugar is reached. This phenomenon
of change of rotation is called as “Mutarotation”.
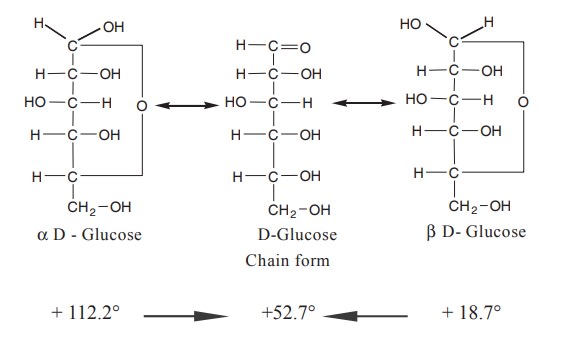
The mutarotation is due to the existence of two
optical isomers of glucose, namely a, D glucose with a specific rotation +112.2°
and b, D glucose with a specific rotation +18.7°
α and β isomers are called as anomers and the carbon
atom responsible for this is the anomeric carbon atom. Anomers are isomers
differing in configuration of a particular carbon atom alone.
A freshly prepared aqueous solution of α, D glucose has a specific rotation of +112.2°. When this solution
is allowed to stand, the rotation falls to 52.7° and remains constant at this
value. This gradual change in specific rotation is called mutarotation.
The value of mutarotation for α, D-glucose is +59.5°.
(+112.2°) - (52.7°) = +59.5°.
A freshly prepared solution of b, D glucose has a rotation value of 18.7°. It also gradually
increases and reaches the same final value of + 52.7°.
Related Topics