Chapter: Biochemistry: Carbohydrates
Disaccharides
Disaccharides
Disaccharides are sugars containing two
molecules of monosaccharides. Disaccharides are formed by the condensation of
two molecules of monosaccharides with the elimination of one molecule of water.
In disaccharides, monosaccharides are linked by
the glycosidic bonds. The properties of the disaccharides depend to a great
extent on the type of linkage. If the two potential aldehyde or ketone of both
monosaccharides are involved in the linkage, the sugar will not exhibit
reducing properties and will not be able to form osazones. eg. Sucrose. But if
one of them is not bound in this way, it will permit reduction and osazone
formation by the sugars eg. lactose and maltose which are known as reducing
disaccharides.
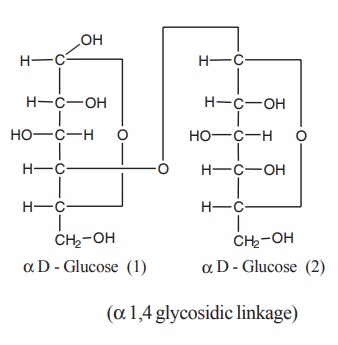
1. Maltose
Maltose is composed of two glucose molecules
combined by a- 1,4 glycosidic linkage. It is commonly called malt sugar. Malt
from sprouting barley is the major source of maltose. It is a rather sweet
sugar and is highly soluble in water.
The structure of maltose shows that the
potential aldehyde group of glucose -2 is blocked in the glycosidic linkage,
whereas the potential aldehyde group of glucose -1 is free and can reduce
alkaline copper solution. It is because of this free aldehyde in the first
glucose molecule, maltose has reducing property.
Metabolism
Maltose is the end product of digestion of
starch by the action of salivary amylase, in the mouth and pancreatic amylase
in the intestine. Maltose is formed as an intermediate product in the
intestine. Maltose is split into two molecules of glucose by the enzyme maltase of the intestinal juice before
absorption.
2. Lactose
Lactose is commonly called as milk sugar. It is
present in the milk of mammals. However lactose is found in the urine of
pregnant and lactating women. It is less soluble in water and less sweeter than
sucrose.
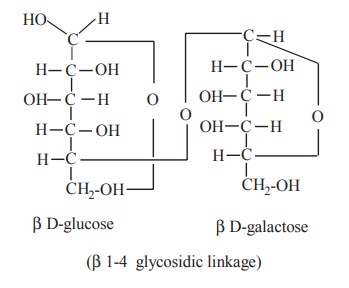
Just as in maltose, lactose has a free
potential adehyde group in the glucose molecule, not involved in the glycosidic
linkage between glucose and galactose molecules. Whereas, the potential
aldehyde group of galactose molecule is blocked in the linkage. Because of the
presence of free aldehyde group in the glucose molecule, lactose can reduce
Fehling’s solution and is therefore a reducing sugar.
Metabolism
When lactose is hydrolysed by acids or by the
enzyme lactase, one molecule of
glucose and one molecule of galactose are formed. The intestine of milk sucking
infants has the enzyme lactase, which converts lactose into glucose and
galactose. Then only it is absorbed in the body. Excess of lactose ingested
into the body causes diarrhoea, abnormal intestinal flow and colic pain. Lactose
is not fermented by yeast.
3. Sucrose
Sucrose is ordinary “table sugar”. It is also
called as “cane sugar” as it can be obtained from sugar cane. It is widely
distributed in sugar cane, beet root, pine apple, honey, carrot and ripe
fruits.
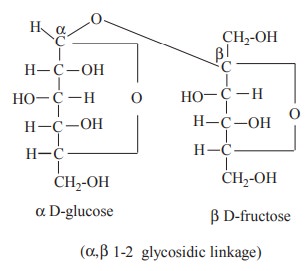
Sucrose consists of one molecule of glucose and
one molecule of fructose. The linkage between these molecules are formed
between the aldehyde group of glucose and the ketone group of fructose. Thus,
both the potential aldehyde group of glucose and the ketone group of fructose
are blocked in the linkage and sucrose has no free reducing group. On account
of this structural peculiarity sucrose is a non-reducing sugar. It does not
reduce Tollen’s and Fehling’s solutions and does not form osazone.
Metabolism
Sucrose on hydrolysis by dilute acids or the
enzyme sucrase or invertase gives a mixture of glucose
and fructose. It is called as invert sugar.
Inversion
Sucrose is dextrorotatory (+62.5°) but it’s
hydrolytic products are levorotatory because fructose has a greater specific
levo-rotation than the dextrorotation of glucose. As the hydrolytic products
inverts the rotation, sucrose is known as invert sugar and the process is
called as invertion. Honey contains plenty of ‘invert sugar’ and the presence
of fructose accounts for the greater sweetness of honey.
Related Topics