Chapter: Biochemistry: Carbohydrates
Chemical properties of glucose
Chemical properties of
glucose
Glucose contain active groups. The active
groups are responsible for their chemical properties. There are three types of
active groups in glucose. They are
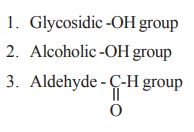
1. Glucoside formation
Glucose reacts with methanol in the presence of
HCl and gives α and β glucoside.
Glucoside formation is due to the reaction of
alcohol with glucoside -OH group of glucose.
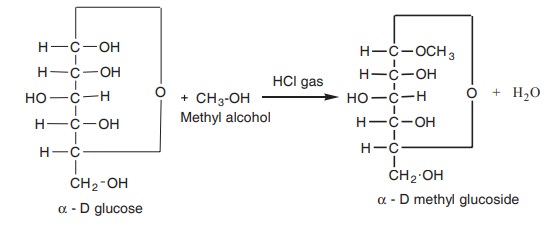
β, D glucose is forms β, D-methyl glucoside. In the same way, fructose forms fructoside.
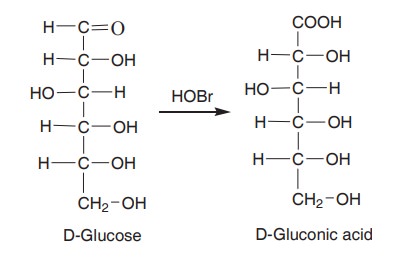
2. Oxidation
Glucose when treated with bromine water, forms
gluconic acid. The aldehyde group is oxidised to carboxylic group.
Br2 + H2O < - - - > HOBr + HBr
First, bromine forms hypobromous acid (HOBr),
with water and oxidises the glucose to gluconic acid.
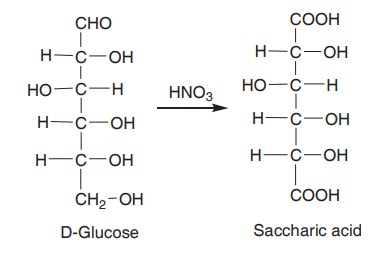
When glucose is oxidised with nitric acid,
saccharic acid is formed.
When glucose is oxidised with hydrogen peroxide
(H2O2), glucuronic acid is formed.
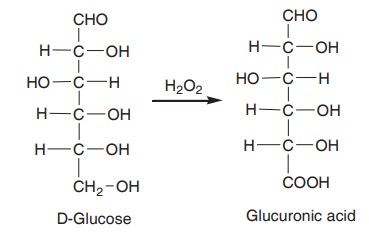
In this reaction only the primary alcohol is
converted into carboxylic group, whereas the aldehyde remains unchanged.
3. Reduction
Monosaccharides can be reduced by various
reducing agents such as sodium-amalgam or by hydrogen under high pressure in
the presence of catalysts. The reduction is due to the presence of aldehyde or
ketone group. On reduction they yield alcohols.
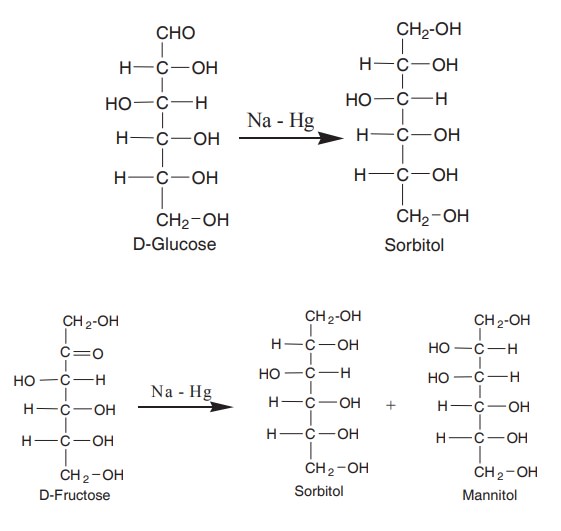
When glucose is reduced by sodium amalgam,
sorbitol is formed. Mannose yeilds mannitol and fructose yeilds a mixture of
sorbitol and mannitol because of the formation of new asymmetric carbon C2
of fructose.
4. Reaction with concentrated H2SO4
Glucose is treated with concentrated H2SO4
or HCl, and forms 5, hydroxymethyl furfural which on further heating yields
levulinic acid and formic acid.
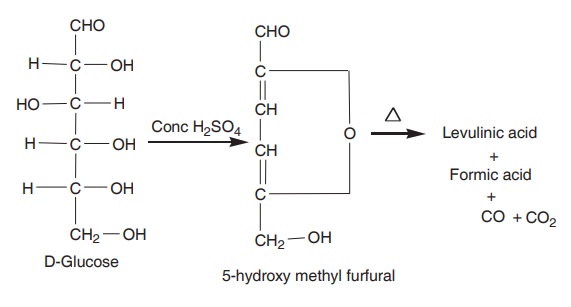
This reaction is the basis of the colour test,
known as Molish test for sugars.
When pentoses are treated with mineral acids furfural is obtained on heating.
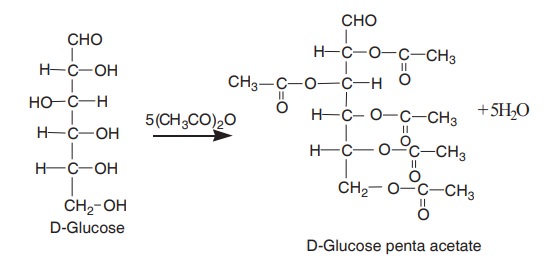
5. Ester formation
They can form esters with carboxylic acids due
to the presence of OH groups. For eg. glucose reacts with five molecules of
acetic anhydride to form penta acetate derivative. It obviously indicates that
the glucose contain five OH groups.
6. Reducing property
Monosaccharides act as the best reducing
agents. They readily reduce oxidizing agents such as ferric cyanide, H2O2
and cupric ion. In such reactions, the sugar is oxidized at the carbonyl group
and the oxidising agent becomes reduced.
Glucose and other sugar capable of reducing
certain compounds are called reducing sugars. Glucose reduces Tollen’s reagent,
Fehling’s reagent, Benedict’s reagent etc. At the same time glucose is oxidized
to gluconic acid.
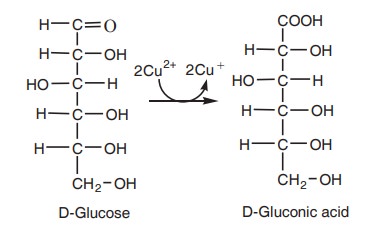
This property is the basis of Fehling’s
reaction (ammoniacal cupric sulphate), a qualitative test for the presence of
reducing sugar.
Cu2+ is reduced into Cu+
and at the same time glucose is oxidised to gluconic acid. During this reaction
the blue colour of the reagent changes to reddish orange colour. Benedict’s
reagent contains cupric ions which are reduced to cuprous ions by the reducing
sugar and the colour change from blue to orange or red. It indicates the
presence of reducing sugar.
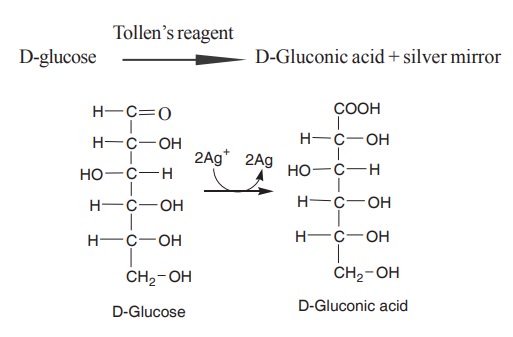
A standard test for the presence of reducing
sugar is the reduction of Ag+ in ammonia solution (Tollen’s reagent) to yield a
metallic silver mirror lining on the sides of the test tube.
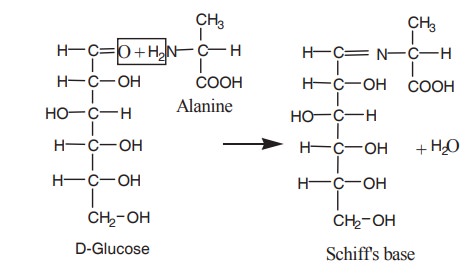
7. Reaction with alanine
The aldehyde group of glucose condenses with
the amino group of alanine to form Schiff’s
base. Fructose also gives Schiff’s base with alanine.
The browning reaction occurs during baking of
bread and other mixtures of carbohydrates and proteins is believed to be due to
the formation of Schiff’s base between the amino groups of proteins and the
aldehyde groups of carbohydrates.
8. Osazone formation
An important reaction of reducing sugars,
(monosaccharide and disaccharrides) having potential aldehyde or ketone group,
is their action with phenylhydrazine to form phenyl hydrazones.
Reaction with phenylhydrazine involves only 2
carbon atoms namely the carbonyl carbon atom and the adjacent one.
The steps involved in phenylhydrazine reactions
are
1. One molecule of glucose condenses with one
molecule of phenyl hydrazine to form soluble glucose phenylhydrazone.
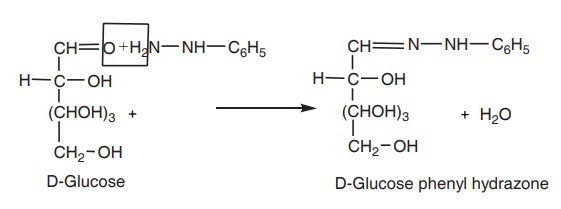
2. In the presence of excess of
phenylhydrazine, another molecule of phenylhydrazine enters the reaction.
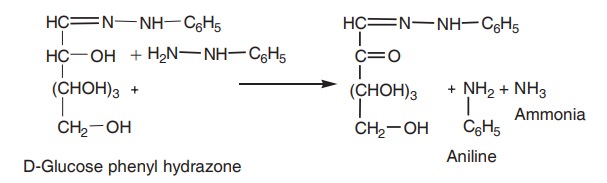
3. Now the third molecule of phenylhydrazine
enters the reaction, giving rise to phenyl glucosazone which are yellow
coloured crystals.
The shape of the crystals and the time of
formation of osazone differ for various sugars.
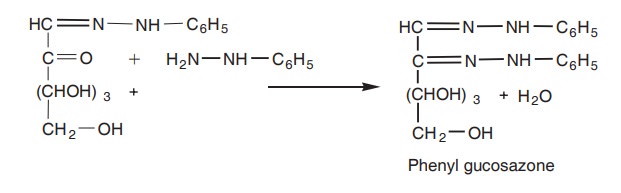
The reaction of phenyl hydrazine with fructose
is similar to glucose. Here again 3 molecules of phenyl hydrazine take part in
the reaction. Fructose gives fructosazone. Disaccharides such as maltose and
lactose also exhibit the property of osazone formation. But, sucrose does not
form osazone, since it does not contain free CHO or CO groups which are
responsible for the reducing property.
9. Fermentation
Fermentation is the process of converting a
larger complex molecule into simple molecules by means of enzymes in an
anaerobic condition. The products of the reaction are alcohol and CO2.
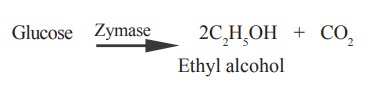
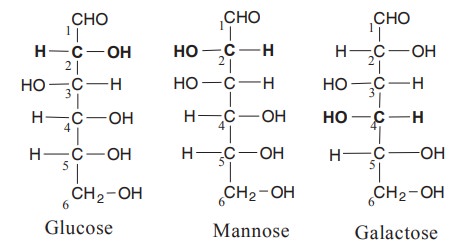
10. Epimerisation
Two sugars which differ from one another only
in configuration around a single carbon atom are termed “epimers”
eg : Glucose and mannose are epimers in respect
of C2. Glucose and galactose differ only with respect to C4.
The process by which one epimer is converted to other is called as epimerization
and it requires the enzyme epimerases in the living organisms. Galactose is
converted to glucose by this manner in our body.
Related Topics