Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Formulation of Biotech Products, Including Biopharmaceutical Considerations
Soluble Carrier Systems for Targeted Delivery of Proteins
Soluble Carrier Systems for Targeted Delivery of Proteins
MAb as Targeted Therapeutic
Agents: Human and Humanized Antibodies
Antibodies are “natural targeting devices.” Their homing ability is
combined with functional activity (Crommelin and Storm, 1990; Crommelin et al.,
1992). MAb can affect the target cell function upon attach-ment. Complement can
be bound via the Fc receptor and subsequently cause lysis of the target cell.
Alternatively, certain Fc receptor-bearing killer cells can induce “antibody
dependent, cell-mediated cyto-toxicity” (ADCC), or contact with macrophages can
be established. Moreover, metabolic deficiencies can beinduced in the target
cells through a blockade of certain essential cell surface receptors by MAb.
A problem that occurs when using murine antibodies for therapy is the
production of human anti-mouse antibodies (HAMA) after administration. HAMA
induction may prohibit further use of these therapeutic MAb by neutralizing the
antigen-binding site; anaphylactic reactions are relatively rare. Concurrent
administration of immunosuppressive agents is a strategy to minimize side
effects. More in depth information regarding immunogenicity of therapeutic
proteins is provided.
There are several other ways to cope with this MAb-induced
immunogenicity problem.. Here, a brief summary of the options relevant for
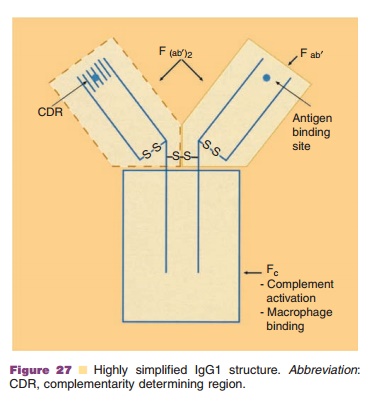
protein targeting suffices. First of all, the use of F(ab)2 or F(ab) fragments (Fig. 27)
avoids raising an immune response against the Fc part, but the development of
humanized or human MAb minimizes the induction of HAMA even further. For
humanization of MAb several options can be considered. One can build chimeric
(partly human, partly murine) molecules consisting of a human Fc part and a
murine Fab part, with the antigen binding sites or, alternatively, only the six
complementarity determining regions (CDRs) of the murine antibody can be
grafted in a human antibody structure. CDR grafting minimizes the exposure to
murine material.
Completely human MAb can be produced by transfecting human antibody
genes into mouse cells, which subsequently produce the human MAb.
Alternatively, transgenic mice can be used. These approaches reduce the
immunogenicity compared to the existing generation of murine MAb. But even with
all these human or humanized MAb, anti-idiotypic immune responses against the
binding site structure of the MAb cannot be excluded.
Bispecific Antibodies
To enhance the therapeutic potential of antibodies, bispecific
antibodies have been designed. Bispecific antibodies are manufactured from two
separate antibodies to create a molecule with two different binding sites
(Fanger and Guyre, 1991). Bispecific MAb bring target cells or tissue (one
antigen-binding site) in contact with other structures (second antigen binding
site). This second antigen binding site can bind to effector cells via
cytotoxicity triggering molecules on T-cells, NK(natural killer) cells, or
macrophages, and thus trigger cytotoxicity.
Bispecific antibodies have been used experi-mentally in the clinic, for instance,
to direct intraper-itoneally injected autologous T-lymphocytes, stimulated with
recombinant interleukin-2, to intra-peritoneally located ovarian carcinoma
cells. This MAb combines an antigen-binding site for a carcino-ma-surface
antigen with an antigen-binding site with T-cell affinity. The MAb are in vitro
incubated with the stimulated T-lymphocytes prior to IP injection (Crommelin et
al., 1992; De Leij et al., 1990).
Immuno conjugates:
Combinations between an Antibody and an Active Compound
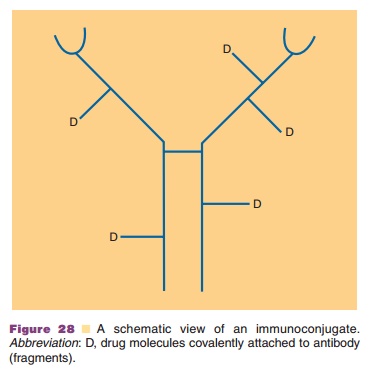
In many cases antibodies alone or bispecific anti-bodies have been shown
to lack sufficient therapeutic activity. To enhance their activity, conjugates
of MAb and drugs have been designed (Fig. 28). These efforts mainly focus on
the treatment of cancer (Crommelin and Storm, 1990). To test the concept of
immunocon-jugates, a wide range of drugs has been covalently bound to
antibodies and has been evaluated in animal tumor models. As only a limited
number of antibody molecules can bind to the target cells, only conjuga-tion of
highly potent drugs will lead to sufficient therapeutic activity. Gemtuzumab
ozogamicin (Mylotarg ) is a conjugate of a monoclonal antibody and
calicheamicin . The MAb part targets the CD33 surface antigen in CD33-positive
acute myeloid leukemia cells (AML). After internali-zation into the cell the
highly cytotoxic calicheamicin is released.
Cytostatics with a high intrinsic cytotoxicity are needed (see above). Because the kinetic behavior of active compounds is strongly affected by the conjugating antibody, not only existing cytostatics, but also active compounds that were never used before as drugs, because of their high toxicity, should now be reconsidered.
Immunoconjugated toxins are now tested as chemotherapeutic agents to
treat cancer (immunotox-ins). Examples of the toxin family are ricin, abrin,
and diphtheria toxin (Fig. 29). These proteins are extre-mely toxic; they block
enzymatically intracellular protein synthesis at the ribosomal level. Ricin (MW
66 kDa) consists of an A and a B chain that are linked through a cystin bridge.
The A chain is responsible for blocking protein synthesis at the ribosomes. The
B chain is important for cellular uptake of the molecule (endocytosis) and
intracellular trafficking.

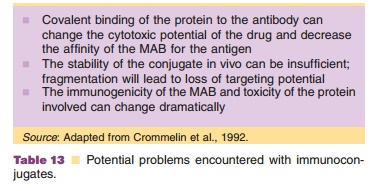
Table 13 lists a number of potential problems encountered with
toxin-based immunoconjugates (Crommelin et al., 1992). In animal studies with
immunoconjugated ricin only a small fraction of these immunotoxins accumulates
in tumor tissue (1%). A major fraction still ends up in the liver, the main
target organ for “natural” ricin. Moreover, in clinical phase I studies (to
assess the safety of the conjugates) the first generation of immunoconjugates
turned out to be immunogenic. Now, attempts are being made to adapt the ricin
molecule (by genetic engineering) so that liver targeting is being mini-mized.
This can be done by blocking (removing or masking) on the ricin molecule
ligands for galactose receptors on hepatocytes. Besides, murine MAb can be
replaced by human or humanized MAb (see above) (Ramakrishnan, 1990).
Related Topics