Home | | Pharmaceutical Biotechnology: Fundamentals and Applications | Shelf Life of Protein Based Pharmaceuticals - Excipients Used in Parenteral Formulations of biotech Products
Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Formulation of Biotech Products, Including Biopharmaceutical Considerations
Shelf Life of Protein Based Pharmaceuticals - Excipients Used in Parenteral Formulations of biotech Products
Proteins can be stored (i) as an aqueous solution, (ii) in freeze-dried form, (iii) in dried form in a compacted state (tablet).
Shelf Life of Protein-Based Pharmaceuticals
Proteins can be stored (i) as an aqueous solution, (ii) in freeze-dried form, (iii) in dried form in a compacted state (tablet). Some mechanisms behind chemical and physical degradation processes have been briefly discussed.
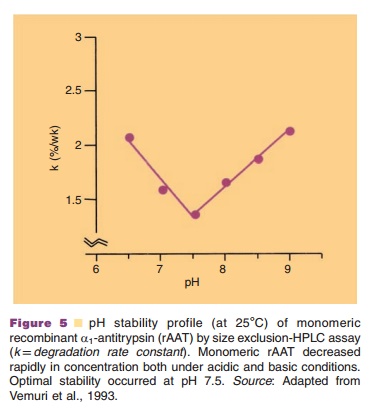
Stability of protein solutions strongly depends on factors such as pH, ionic strength, temperature, and the presence of stabilizers. For example, Figure 5 shows the pH dependence of a1-antitrypsin and clearly demonstrates the critical importance of pH for the shelf life of proteins.
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
Pharmaceutical Biotechnology: Fundamentals and Applications : Formulation of Biotech Products, Including Biopharmaceutical Considerations : Shelf Life of Protein Based Pharmaceuticals - Excipients Used in Parenteral Formulations of biotech Products |
Related Topics
Pharmaceutical Biotechnology: Fundamentals and Applications : Formulation of Biotech Products, Including Biopharmaceutical Considerations