Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Formulation of Biotech Products, Including Biopharmaceutical Considerations
Delivery of Proteins: Site Specific Delivery (Targeting) of Protein Drugs
SITE-SPECIFIC DELIVERY (TARGETING) OF PROTEIN DRUGS
Why are we still not able to beat life-threatening diseases such as
cancer with our current arsenal of drugs? Causes of failure can be summarized
as follows (Crommelin et al., 1992):
(1) The active compound never reaches the target site, because it is rapidly
eliminated intact from the body through the kidneys, or it is inactivated
through metabolic action (e.g., in the liver).
(3) Only a small fraction of the drug reaches the target site. By far the largest
fraction of the drug is distributed over non-target organs, where they exert
side effects; in other words, accumulation of the drug at the target site is
the exceptionMany drug molecules (in particular high MW and hydrophilic
molecules, i.e., many therapeu-tic proteins) do not enter cells easily. This
poses a problem if intracellular delivery is required for their therapeutic
activity.
Attempts are made to increase the therapeutic index of drugs through
drug targeting:
(1) by specific delivery of the active compound to its site of action,
(2) to keep it there until it has been inactivated and detoxified.
Targeted drug delivery should maximize the therapeutic effect and avoid
toxic effects elsewhere. The basics of the concept of drug targeting were
defined already in the early days of the 20th century by Paul Ehrlich. But only
in the last decade substantial progress has been made to implement this
site-specific delivery concept. Recent progress can be ascribed to: (i) the rapidly growing number of technological options (e.g., safe
carriers) for drug delivery; (ii) many new insights gained into
the pathophysiology of diseases at the cellular and molecular level, including
the presence of cell-specific receptors and homing devices to target them
(e.g., MAb); and finally, (iii) new revelations on the nature
of the anatomical and physiological barriers that hinder easy access to target
sites. The site-specific delivery systems presently in different stages of
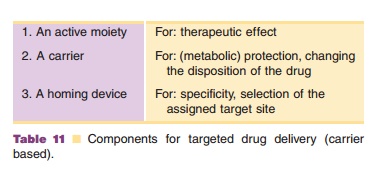
development generally consist of three functionally separate units (Table 11).
Nature has provided us with antibodies, which exemplify a class of
natural drug targeting devices. In an antibody molecule one can recognize a
homing device part (antigen binding site) and “active” parts. These active
parts in the molecule are responsible for participating in the complement
cascade, or inducing interactions with monocytes when antigen is bound. The
rest of the molecule can be considered as carrier.
Most of the drug (protein) targeting work is performed with delivery systems that are designed for parenteral and, more specifically, IV delivery. Only a limited number of papers have dealt with the pharmacokinetics of the drug targeting process (Hunt et al., 1986). From these kinetic models a number of conclusions could be drawn for situations where targeted delivery is, in principle, advantageous (Table 12).

The potential and limitations of carrier-based, targeted drug delivery
systems for proteins are briefly discussed. The focus is on concepts where MAb
are being used. They can be used as the antibody itself, in modified form when
anti-bodies are conjugated with an active moiety, or attached to drug-laden colloidal
carriers such as liposomes.
Two terms are regularly used in the context of targeting: passive and
active targeting. With passive targeting the “natural” disposition pattern of
the carrier system is utilized for site-specific delivery. For instance, particulate
carriers circulating in the blood (see below) are often rapidly taken up by
macrophages in contact with the blood circulation and accumulate in liver
(Kupffer cells) and spleen. Active targeting is the concept where attempts are
made to change the natural disposition of the carrier by some sort of homing
device or homing principle to select one particular tissue or cell type.
Related Topics